Zooming in on the intracellular microbiome composition of bacterivorous Acanthamoeba isolates
- PMID: 38500701
- PMCID: PMC10945361
- DOI: 10.1093/ismeco/ycae016
Zooming in on the intracellular microbiome composition of bacterivorous Acanthamoeba isolates
Abstract
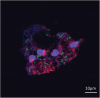
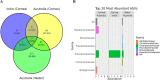
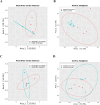
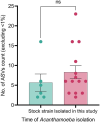
Acanthamoeba, a free-living amoeba in water and soil, is an emerging pathogen causing severe eye infection known as Acanthamoeba keratitis. In its natural environment, Acanthamoeba performs a dual function as an environmental heterotrophic predator and host for a range of microorganisms that resist digestion. Our objective was to characterize the intracellular microorganisms of phylogenetically distinct Acanthamoeba spp. isolated in Australia and India through directly sequencing 16S rRNA amplicons from the amoebae. The presence of intracellular bacteria was further confirmed by in situ hybridization and electron microscopy. Among the 51 isolates assessed, 41% harboured intracellular bacteria which were clustered into four major phyla: Pseudomonadota (previously known as Proteobacteria), Bacteroidota (previously known as Bacteroidetes), Actinomycetota (previously known as Actinobacteria), and Bacillota (previously known as Firmicutes). The linear discriminate analysis effect size analysis identified distinct microbial abundance patterns among the sample types; Pseudomonas species was abundant in Australian corneal isolates (P < 0.007), Enterobacteriales showed higher abundance in Indian corneal isolates (P < 0.017), and Bacteroidota was abundant in Australian water isolates (P < 0.019). The bacterial beta diversity of Acanthamoeba isolates from keratitis patients in India and Australia significantly differed (P < 0.05), while alpha diversity did not vary based on the country of origin or source of isolation (P > 0.05). More diverse intracellular bacteria were identified in water isolates as compared with clinical isolates. Confocal and electron microscopy confirmed the bacterial cells undergoing binary fission within the amoebal host, indicating the presence of viable bacteria. This study sheds light on the possibility of a sympatric lifestyle within Acanthamoeba, thereby emphasizing its crucial role as a bunker and carrier of potential human pathogens.
Keywords: Acanthamoeba; environmental predator; eye infection; sympatric lifestyle; training ground.
© The Author(s) 2024. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
The role of naturally acquired intracellular Pseudomonas aeruginosa in the development of Acanthamoeba keratitis in an animal model.PLoS Negl Trop Dis. 2024 Jan 2;18(1):e0011878. doi: 10.1371/journal.pntd.0011878. eCollection 2024 Jan. PLoS Negl Trop Dis. 2024. PMID: 38166139 Free PMC article.
-
Diversity of free-living amoebae in soils and their associated human opportunistic bacteria.Parasitol Res. 2017 Nov;116(11):3151-3162. doi: 10.1007/s00436-017-5632-6. Epub 2017 Oct 7. Parasitol Res. 2017. PMID: 28988383
-
Characterization of the bacterial microbiomes of social amoebae and exploration of the roles of host and environment on microbiome composition.Environ Microbiol. 2021 Jan;23(1):126-142. doi: 10.1111/1462-2920.15279. Epub 2020 Nov 3. Environ Microbiol. 2021. PMID: 33063404
-
A Systematic Review of Intracellular Microorganisms within Acanthamoeba to Understand Potential Impact for Infection.Pathogens. 2021 Feb 18;10(2):225. doi: 10.3390/pathogens10020225. Pathogens. 2021. PMID: 33670718 Free PMC article. Review.
-
Acanthamoeba spp. as a universal host for pathogenic microorganisms: One bridge from environment to host virulence.Microbiol Res. 2016 Dec;193:30-38. doi: 10.1016/j.micres.2016.08.001. Epub 2016 Aug 2. Microbiol Res. 2016. PMID: 27825484 Review.
Cited by
-
Morphological Characterization and Genotyping of Acanthamoeba Isolates From Oral and Nasal Samples of Cancer Patients in Kashan, Iran.Can J Infect Dis Med Microbiol. 2024 Nov 13;2024:4071707. doi: 10.1155/2024/4071707. eCollection 2024. Can J Infect Dis Med Microbiol. 2024. PMID: 39569149 Free PMC article.
-
Unravelling mechanisms of bacterial recognition by Acanthamoeba: insights into microbial ecology and immune responses.Front Microbiol. 2024 Aug 23;15:1405133. doi: 10.3389/fmicb.2024.1405133. eCollection 2024. Front Microbiol. 2024. PMID: 39247694 Free PMC article. Review.
References
-
- Antonelli A, Favuzza E, Galano Aet al. . Regional spread of contact lens-related Acanthamoeba keratitis in Italy. New Microbiol 2018;41:83–5. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

