Role and molecular mechanisms of SGLT2 inhibitors in pathological cardiac remodeling (Review)
- PMID: 38488029
- PMCID: PMC10955520
- DOI: 10.3892/mmr.2024.13197
Role and molecular mechanisms of SGLT2 inhibitors in pathological cardiac remodeling (Review)
Abstract
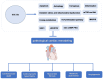
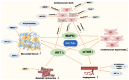
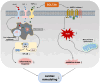
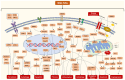
Cardiovascular diseases are caused by pathological cardiac remodeling, which involves fibrosis, inflammation and cell dysfunction. This includes autophagy, apoptosis, oxidative stress, mitochondrial dysfunction, changes in energy metabolism, angiogenesis and dysregulation of signaling pathways. These changes in heart structure and/or function ultimately result in heart failure. In an effort to prevent this, multiple cardiovascular outcome trials have demonstrated the cardiac benefits of sodium‑glucose cotransporter type 2 inhibitors (SGLT2is), hypoglycemic drugs initially designed to treat type 2 diabetes mellitus. SGLT2is include empagliflozin and dapagliflozin, which are listed as guideline drugs in the 2021 European Guidelines for Heart Failure and the 2022 American Heart Association/American College of Cardiology/Heart Failure Society of America Guidelines for Heart Failure Management. In recent years, multiple studies using animal models have explored the mechanisms by which SGLT2is prevent cardiac remodeling. This article reviews the role of SGLT2is in cardiac remodeling induced by different etiologies to provide a guideline for further evaluation of the mechanisms underlying the inhibition of pathological cardiac remodeling by SGLT2is, as well as the development of novel drug targets.
Keywords: SGLT2 inhibitors; cardiac fibroblasts; cardiac remodeling; molecular mechanisms; myocardial hypertrophy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
The effect of sodium-glucose cotransporter-2 inhibitors on cardiac structure remodeling and function: A meta-analysis of randomized controlled trials.Eur J Intern Med. 2023 Aug;114:49-57. doi: 10.1016/j.ejim.2023.04.002. Epub 2023 Apr 14. Eur J Intern Med. 2023. PMID: 37062643
-
New insights into the molecular mechanisms of SGLT2 inhibitors on ventricular remodeling.Int Immunopharmacol. 2023 May;118:110072. doi: 10.1016/j.intimp.2023.110072. Epub 2023 Apr 3. Int Immunopharmacol. 2023. PMID: 37018976 Review.
-
Sodium-Glucose Cotransporter 2 Inhibitors and Cardiac Remodeling.J Cardiovasc Transl Res. 2022 Oct;15(5):944-956. doi: 10.1007/s12265-022-10220-5. Epub 2022 Mar 15. J Cardiovasc Transl Res. 2022. PMID: 35290593 Review.
-
Use of Sodium-Glucose Cotransporter-2 Inhibitors in Clinical Practice for Heart Failure Prevention and Treatment: Beyond Type 2 Diabetes. A Narrative Review.Adv Ther. 2022 Feb;39(2):845-861. doi: 10.1007/s12325-021-01989-z. Epub 2021 Dec 9. Adv Ther. 2022. PMID: 34881413 Free PMC article. Review.
-
Sodium-glucose Cotransporter 2 Inhibitors and Pathological Myocardial Hypertrophy.Curr Drug Targets. 2023;24(13):1009-1022. doi: 10.2174/1389450124666230907115831. Curr Drug Targets. 2023. PMID: 37691190 Free PMC article.
Cited by
-
The role of GZMA as a target of cysteine and biomarker in Alzheimer's disease, pelvic organ prolapse, and tumor progression.Front Pharmacol. 2024 Aug 20;15:1447605. doi: 10.3389/fphar.2024.1447605. eCollection 2024. Front Pharmacol. 2024. PMID: 39228516 Free PMC article.
-
Heart Failure with Preserved Ejection Fraction and Cardiac Amyloidosis in the Aging Heart.Int J Mol Sci. 2024 Oct 26;25(21):11519. doi: 10.3390/ijms252111519. Int J Mol Sci. 2024. PMID: 39519069 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

