Epigenetic Regulation of Autophagy in Bone Metabolism
- PMID: 38486976
- PMCID: PMC10935486
- DOI: 10.1093/function/zqae004
Epigenetic Regulation of Autophagy in Bone Metabolism
Abstract
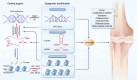
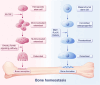
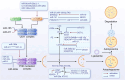
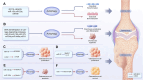
The skeletal system is crucial for supporting bodily functions, protecting vital organs, facilitating hematopoiesis, and storing essential minerals. Skeletal homeostasis, which includes aspects such as bone density, structural integrity, and regenerative processes, is essential for normal skeletal function. Autophagy, an intricate intracellular mechanism for degrading and recycling cellular components, plays a multifaceted role in bone metabolism. It involves sequestering cellular waste, damaged proteins, and organelles within autophagosomes, which are then degraded and recycled. Autophagy's impact on bone health varies depending on factors such as regulation, cell type, environmental cues, and physiological context. Despite being traditionally considered a cytoplasmic process, autophagy is subject to transcriptional and epigenetic regulation within the nucleus. However, the precise influence of epigenetic regulation, including DNA methylation, histone modifications, and non-coding RNA expression, on cellular fate remains incompletely understood. The interplay between autophagy and epigenetic modifications adds complexity to bone cell regulation. This article provides an in-depth exploration of the intricate interplay between these two regulatory paradigms, with a focus on the epigenetic control of autophagy in bone metabolism. Such an understanding enhances our knowledge of bone metabolism-related disorders and offers insights for the development of targeted therapeutic strategies.
Keywords: autophagy; bone homeostasis; bone metabolism; bone-related diseases; epigenetics; miRNA.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Physiological Society.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Epigenetic regulation of autophagy by histone-modifying enzymes under nutrient stress.Cell Death Differ. 2023 Jun;30(6):1430-1436. doi: 10.1038/s41418-023-01154-9. Epub 2023 Mar 30. Cell Death Differ. 2023. PMID: 36997734 Free PMC article. Review.
-
Revealing the role of epigenetic and post-translational modulations of autophagy proteins in the regulation of autophagy and cancer: a therapeutic approach.Mol Biol Rep. 2023 Dec 8;51(1):3. doi: 10.1007/s11033-023-08961-w. Mol Biol Rep. 2023. PMID: 38063905 Review.
-
Navigating the autophagic landscape: Epigenetic modulation in gastrointestinal cancer.World J Gastroenterol. 2024 Aug 21;30(31):3628-3634. doi: 10.3748/wjg.v30.i31.3628. World J Gastroenterol. 2024. PMID: 39192999 Free PMC article.
-
Epigenetic Regulation of Autophagy.Adv Exp Med Biol. 2019;1206:221-236. doi: 10.1007/978-981-15-0602-4_11. Adv Exp Med Biol. 2019. PMID: 31776988 Review.
-
Molecular mechanisms in regulation of autophagy and apoptosis in view of epigenetic regulation of genes and involvement of liquid-liquid phase separation.Cancer Lett. 2024 Apr 10;587:216779. doi: 10.1016/j.canlet.2024.216779. Epub 2024 Mar 6. Cancer Lett. 2024. PMID: 38458592 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
