Lysosomes in Cancer-At the Crossroad of Good and Evil
- PMID: 38474423
- PMCID: PMC10930463
- DOI: 10.3390/cells13050459
Lysosomes in Cancer-At the Crossroad of Good and Evil
Abstract
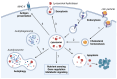
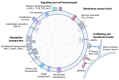
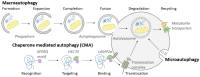
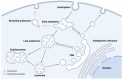
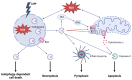
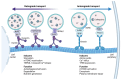
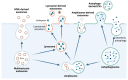
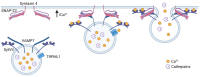
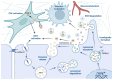
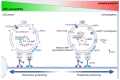
Although it has been known for decades that lysosomes are central for degradation and recycling in the cell, their pivotal role as nutrient sensing signaling hubs has recently become of central interest. Since lysosomes are highly dynamic and in constant change regarding content and intracellular position, fusion/fission events allow communication between organelles in the cell, as well as cell-to-cell communication via exocytosis of lysosomal content and release of extracellular vesicles. Lysosomes also mediate different forms of regulated cell death by permeabilization of the lysosomal membrane and release of their content to the cytosol. In cancer cells, lysosomal biogenesis and autophagy are increased to support the increased metabolism and allow growth even under nutrient- and oxygen-poor conditions. Tumor cells also induce exocytosis of lysosomal content to the extracellular space to promote invasion and metastasis. However, due to the enhanced lysosomal function, cancer cells are often more susceptible to lysosomal membrane permeabilization, providing an alternative strategy to induce cell death. This review summarizes the current knowledge of cancer-associated alterations in lysosomal structure and function and illustrates how lysosomal exocytosis and release of extracellular vesicles affect disease progression. We focus on functional differences depending on lysosomal localization and the regulation of intracellular transport, and lastly provide insight how new therapeutic strategies can exploit the power of the lysosome and improve cancer treatment.
Keywords: LMP; exocytosis; extracellular vesicles; lysosomal positioning; lysosome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Histology, Axon.2022 Nov 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2022 Nov 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 32119275 Free Books & Documents.
-
Peer Play.2023 Jul 4. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2023 Jul 4. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30020595 Free Books & Documents.
-
Healthcare workers' informal uses of mobile phones and other mobile devices to support their work: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2024 Aug 27;8(8):CD015705. doi: 10.1002/14651858.CD015705.pub2. Cochrane Database Syst Rev. 2024. PMID: 39189465 Free PMC article.
-
Underlying Mechanism of Lysosomal Membrane Permeabilization in CNS Injury: A Literature Review.Mol Neurobiol. 2025 Jan;62(1):626-642. doi: 10.1007/s12035-024-04290-6. Epub 2024 Jun 18. Mol Neurobiol. 2025. PMID: 38888836 Review.
Cited by
-
Lysosome-Associated Membrane Protein-3 (LAMP3) Expression in Oral Squamous Cell Carcinoma and Its Relationship With Clinicopathological Parameters: A Cross-Sectional Study.Cureus. 2024 Sep 20;16(9):e69790. doi: 10.7759/cureus.69790. eCollection 2024 Sep. Cureus. 2024. PMID: 39429383 Free PMC article.
-
NKX3-2 Induces Ovarian Cancer Cell Migration by HDAC6-Mediated Repositioning of Lysosomes and Inhibition of Autophagy.Cells. 2024 Nov 4;13(21):1816. doi: 10.3390/cells13211816. Cells. 2024. PMID: 39513923 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

