Human Macrophages Activate Bystander Neutrophils' Metabolism and Effector Functions When Challenged with Mycobacterium tuberculosis
- PMID: 38474145
- PMCID: PMC10932022
- DOI: 10.3390/ijms25052898
Human Macrophages Activate Bystander Neutrophils' Metabolism and Effector Functions When Challenged with Mycobacterium tuberculosis
Abstract
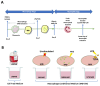
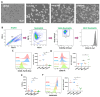
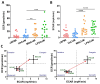
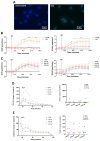
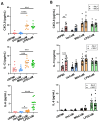
Neutrophils are dynamic cells, playing a critical role in pathogen clearance; however, neutrophil infiltration into the tissue can act as a double-edged sword. They are one of the primary sources of excessive inflammation during infection, which has been observed in many infectious diseases including pneumonia and active tuberculosis (TB). Neutrophil function is influenced by interactions with other immune cells within the inflammatory lung milieu; however, how these interactions affect neutrophil function is unclear. Our study examined the macrophage-neutrophil axis by assessing the effects of conditioned medium (MΦ-CM) from primary human monocyte-derived macrophages (hMDMs) stimulated with LPS or a whole bacterium (Mycobacterium tuberculosis) on neutrophil function. Stimulated hMDM-derived MΦ-CM boosts neutrophil activation, heightening oxidative and glycolytic metabolism, but diminishes migratory potential. These neutrophils exhibit increased ROS production, elevated NET formation, and heightened CXCL8, IL-13, and IL-6 compared to untreated or unstimulated hMDM-treated neutrophils. Collectively, these data show that MΦ-CM from stimulated hMDMs activates neutrophils, bolsters their energetic profile, increase effector and inflammatory functions, and sequester them at sites of infection by decreasing their migratory capacity. These data may aid in the design of novel immunotherapies for severe pneumonia, active tuberculosis and other diseases driven by pathological inflammation mediated by the macrophage-neutrophil axis.
Keywords: Mycobacterium tuberculosis; glycolysis; granulocytes; immunometabolism; infection; neutrophil function; neutrophil metabolism; neutrophil priming and activation; polymorphonuclear cells; tuberculosis.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Lactate Alters Metabolism in Human Macrophages and Improves Their Ability to Kill Mycobacterium tuberculosis.Front Immunol. 2021 Oct 6;12:663695. doi: 10.3389/fimmu.2021.663695. eCollection 2021. Front Immunol. 2021. PMID: 34691015 Free PMC article.
-
Fatty acid metabolism in neutrophils promotes lung damage and bacterial replication during tuberculosis.PLoS Pathog. 2024 Oct 4;20(10):e1012188. doi: 10.1371/journal.ppat.1012188. eCollection 2024 Oct. PLoS Pathog. 2024. PMID: 39365825 Free PMC article.
-
Mycobacterium tuberculosis- induced neutrophil extracellular traps activate human macrophages.J Innate Immun. 2013;5(6):591-602. doi: 10.1159/000348676. Epub 2013 Apr 26. J Innate Immun. 2013. PMID: 23635526 Free PMC article.
-
Neutrophils in Tuberculosis-Associated Inflammation and Lung Pathology.Front Immunol. 2020 May 27;11:962. doi: 10.3389/fimmu.2020.00962. eCollection 2020. Front Immunol. 2020. PMID: 32536917 Free PMC article. Review.
-
Defining the role of neutrophils in the lung during infection: Implications for tuberculosis disease.Front Immunol. 2022 Sep 20;13:984293. doi: 10.3389/fimmu.2022.984293. eCollection 2022. Front Immunol. 2022. PMID: 36203565 Free PMC article. Review.
References
-
- Ong C.W.M., Elkington P.T., Brilha S., Ugarte-Gil C., Tome-Esteban M.T., Tezera L.B., Pabisiak P.J., Moores R.C., Sathyamoorthy T., Patel V., et al. Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis. PLoS Pathog. 2015;11:e1004917. doi: 10.1371/journal.ppat.1004917. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

