The Efficacy and Safety of Immune Checkpoint Inhibitors in Adrenocortical Carcinoma: A Systematic Review and Meta-Analysis
- PMID: 38473262
- PMCID: PMC10931182
- DOI: 10.3390/cancers16050900
The Efficacy and Safety of Immune Checkpoint Inhibitors in Adrenocortical Carcinoma: A Systematic Review and Meta-Analysis
Abstract
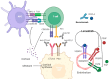
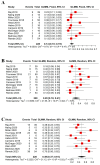
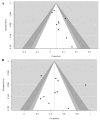
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of different malignancies. However, their efficacy in advanced adrenocortical carcinoma (ACC) remains uncertain. Thus, we conducted a systematic review and meta-analysis to summarize the efficacy and tolerability of ICIs in patients with advanced ACC. We searched PubMed, Scopus, and CENTRAL for studies that used ICIs in ACC. Studies with more than five patients were included in the meta-analysis of the objective response rate (ORR), disease control rate (DCR), overall survival (OS), progression-free survival (PFS), and grade 3/4 adverse events. Twenty studies with 23 treatment arms and 250 patients were included. Single-agent anti-PD1 or anti-PD-L1 treatment was utilized in 13 treatment arms, whereas an anti-PD1 or anti-PD-L1 and anti-CTLA4 combination was used in 4 treatment arms. Other anti-PD1- or anti-PD-L1-based combinations were used in five treatment arms. The ORR was 14% (95% CI = 10-19%, I2 = 0%), and the DCR was 43% (95% CI = 37-50%, I2 = 13%). The combination anti-PD1- or anti-PD-L1-based treatment strategies did not correlate with higher responses compared with monotherapy. The median OS was 13.9 months (95% CI = 7.85-23.05), and the median PFS was 2.8 months (95% CI = 1.8-5.4). ICIs have a modest efficacy in advanced ACC but a good OS. Further studies are needed to investigate predictive biomarkers for ICI response and to compare ICI-based strategies with the current standard of care.
Keywords: adrenocortical carcinoma; immune checkpoint inhibitors; immunotherapy; meta-analysis.
Conflict of interest statement
Aditya Shreenivas is an advisory board member for Taiho Oncology and Iylon Pvt. Ltd., receives research support from Natera and Caris, and is a member of the virtual tumor board Target Cancer Foundation. All the other authors declare no conflicts of interest.
Figures


Similar articles
-
Efficacy and safety of immune checkpoint inhibitors for advanced non-small cell lung cancer with or without PD-L1 selection: A systematic review and network meta-analysis.Chin Med J (Engl). 2023 Sep 20;136(18):2156-2165. doi: 10.1097/CM9.0000000000002750. Epub 2023 Aug 18. Chin Med J (Engl). 2023. PMID: 37596898 Free PMC article.
-
Benefits of combination therapy with immune checkpoint inhibitors and predictive role of tumour mutation burden in hepatocellular carcinoma: A systematic review and meta-analysis.Int Immunopharmacol. 2022 Nov;112:109244. doi: 10.1016/j.intimp.2022.109244. Epub 2022 Sep 18. Int Immunopharmacol. 2022. PMID: 36126410 Review.
-
Anti-PD1 versus anti-PD-L1 immunotherapy in first-line therapy for advanced non-small cell lung cancer: A systematic review and meta-analysis.Thorac Cancer. 2021 Apr;12(7):1058-1066. doi: 10.1111/1759-7714.13867. Epub 2021 Feb 14. Thorac Cancer. 2021. PMID: 33586297 Free PMC article.
-
Efficacy and safety of immune checkpoint inhibitors in recurrent or metastatic head and neck squamous cell carcinoma: A systematic review and meta-analysis of randomized clinical trials.Cancer Med. 2023 Oct;12(20):20277-20286. doi: 10.1002/cam4.6564. Epub 2023 Oct 10. Cancer Med. 2023. PMID: 37814950 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article. Review.
References
-
- Fassnacht M., Dekkers O.M., Else T., Baudin E., Berruti A., de Krijger R.R., Haak H.R., Mihai R., Assie G., Terzolo M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018;179:G1–G46. doi: 10.1530/EJE-18-0608. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

