An integrative mechanistic model of thymocyte dynamics
- PMID: 38469297
- PMCID: PMC10925769
- DOI: 10.3389/fimmu.2024.1321309
An integrative mechanistic model of thymocyte dynamics
Abstract
Background: The thymus plays a central role in shaping human immune function. A mechanistic, quantitative description of immune cell dynamics and thymic output under homeostatic conditions and various patho-physiological scenarios are of particular interest in drug development applications, e.g., in the identification of potential therapeutic targets and selection of lead drug candidates against infectious diseases.
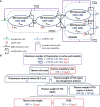
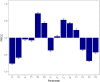
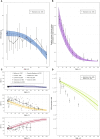
Methods: We here developed an integrative mathematical model of thymocyte dynamics in human. It incorporates mechanistic features of thymocyte homeostasis as well as spatial constraints of the thymus and considerations of age-dependent involution. All model parameter estimates were obtained based on published physiological data of thymocyte dynamics and thymus properties in mouse and human. We performed model sensitivity analyses to reveal potential therapeutic targets through an identification of processes critically affecting thymic function; we further explored differences in thymic function across healthy subjects, multiple sclerosis patients, and patients on fingolimod treatment.
Results: We found thymic function to be most impacted by the egress, proliferation, differentiation and death rates of those thymocytes which are most differentiated. Model predictions also showed that the clinically observed decrease in relapse risk with age, in multiple sclerosis patients who would have discontinued fingolimod therapy, can be explained mechanistically by decreased thymic output with age. Moreover, we quantified the effects of fingolimod treatment duration on thymic output.
Conclusions: In summary, the proposed model accurately describes, in mechanistic terms, thymic output as a function of age. It may be further used to perform predictive simulations of clinically relevant scenarios which combine specific patho-physiological conditions and pharmacological interventions of interest.
Keywords: immune system; mechanistic model; thymic involution; thymopoiesis; thymus.
Copyright © 2024 Kulesh, Peskov, Helmlinger and Bocharov.
Conflict of interest statement
Author KP is an employee and shareholder of Modeling & Simulation Decisions FZ - LLC. Author GH is an employee of Biorchestra US, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Modeling & Simulation Decisions FZ - LLC. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Conventional and Computational Flow Cytometry Analyses Reveal Sustained Human Intrathymic T Cell Development From Birth Until Puberty.Front Immunol. 2020 Aug 4;11:1659. doi: 10.3389/fimmu.2020.01659. eCollection 2020. Front Immunol. 2020. PMID: 32849574 Free PMC article.
-
Abrogation of Notch Signaling in Embryonic TECs Impacts Postnatal mTEC Homeostasis and Thymic Involution.Front Immunol. 2022 May 30;13:867302. doi: 10.3389/fimmu.2022.867302. eCollection 2022. Front Immunol. 2022. PMID: 35707539 Free PMC article.
-
Gender Disparity Impacts on Thymus Aging and LHRH Receptor Antagonist-Induced Thymic Reconstitution Following Chemotherapeutic Damage.Front Immunol. 2020 Mar 3;11:302. doi: 10.3389/fimmu.2020.00302. eCollection 2020. Front Immunol. 2020. PMID: 32194555 Free PMC article.
-
Cytokines in the thymus: production and biological effects.Curr Med Chem. 2004 Feb;11(4):447-64. doi: 10.2174/0929867043455972. Curr Med Chem. 2004. PMID: 14965226 Review.
-
The thymus gland: a target organ for growth hormone.Scand J Immunol. 2002 May;55(5):442-52. doi: 10.1046/j.1365-3083.2002.01077.x. Scand J Immunol. 2002. PMID: 11975755 Review.
References
-
- Punt J, Owen JA, Stranford SA, Jones PP. Kuby immunology. Eighth edition. New York: W.H. Freeman/Macmillan Learning; (2019). Kuby Janis.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

