Immune checkpoint inhibitors and acute kidney injury
- PMID: 38464524
- PMCID: PMC10920224
- DOI: 10.3389/fimmu.2024.1353339
Immune checkpoint inhibitors and acute kidney injury
Abstract
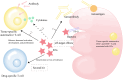
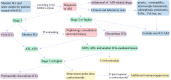
As a new type of anti-tumor immunotherapy, immune checkpoint inhibitors (ICIs) have improved the prognosis of multiple malignancies. However, renal complications are becoming more frequent. Nephrotoxicity often manifests as acute kidney injury (AKI), and the most common histopathological type is acute tubulointerstitial nephritis (ATIN). Based on previous studies of the incidence and potential risk factors for nephrotoxicity, in this review, we describe the mechanism of AKI after ICIs treatment, summarize the incidence, risk factors, and outcomes of AKI, and discuss the diagnosis and management of immune checkpoint inhibitors-associated acute kidney injury (ICI-AKI). In addition, we review the current status of ICIs rechallenge and the therapeutic strategies of ICIs applied in kidney transplant recipients. Finally, we emphasize the importance of collaboration between nephrologists and oncologists to guide the treatment of ICIs and the management of renal complications.
Keywords: acute kidney injury; immune checkpoint inhibitors; immune-related adverse events; immunotherapy; malignancies; nephrotoxicity.
Copyright © 2024 Zhou, Gao, Kong, Wang, Si, Han, Li, Lv and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Immune Checkpoint Inhibitor-Associated AKI: Debates in Diagnosis, Management, and Rechallenge.Semin Nephrol. 2022 Nov;42(6):151346. doi: 10.1016/j.semnephrol.2023.151346. Epub 2023 May 1. Semin Nephrol. 2022. PMID: 37137187 Review.
-
Immune checkpoint inhibitor-associated nephritis-treatment standard.Nephrol Dial Transplant. 2024 Oct 30;39(11):1785-1798. doi: 10.1093/ndt/gfae184. Nephrol Dial Transplant. 2024. PMID: 39138117 Review.
-
The Use of Immune Checkpoint Inhibitors in Oncology and the Occurrence of AKI: Where Do We Stand?Front Immunol. 2020 Oct 8;11:574271. doi: 10.3389/fimmu.2020.574271. eCollection 2020. Front Immunol. 2020. PMID: 33162990 Free PMC article. Review.
-
Immune checkpoint inhibitors in patients with chronic kidney disease: Assessing their ability to cause acute kidney injury and informing their proper use.Semin Oncol. 2022 Apr;49(2):141-147. doi: 10.1053/j.seminoncol.2022.01.012. Epub 2022 Feb 15. Semin Oncol. 2022. PMID: 35264308 Review.
-
Immune Checkpoint Inhibitor Nephrotoxicity: Update 2020.Kidney360. 2020 Jan 14;1(2):130-140. doi: 10.34067/KID.0000852019. eCollection 2020 Feb 27. Kidney360. 2020. PMID: 35372904 Free PMC article. Review.
Cited by
-
High-throughput screening for optimizing adoptive T cell therapies.Exp Hematol Oncol. 2024 Nov 13;13(1):113. doi: 10.1186/s40164-024-00580-w. Exp Hematol Oncol. 2024. PMID: 39538305 Free PMC article. Review.
References
-
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. . Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol. (2019) 5:1411–20. doi: 10.1001/jamaoncol.2019.2187. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

