STAP-2 facilitates insulin signaling through binding to CAP/c-Cbl and regulates adipocyte differentiation
- PMID: 38461189
- PMCID: PMC10925025
- DOI: 10.1038/s41598-024-56533-0
STAP-2 facilitates insulin signaling through binding to CAP/c-Cbl and regulates adipocyte differentiation
Abstract
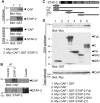
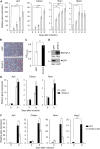
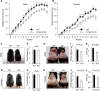
Signal-transducing adaptor protein-2 (STAP-2) is an adaptor molecule involved in several cellular signaling cascades. Here, we attempted to identify novel STAP-2 interacting molecules, and identified c-Cbl associated protein (CAP) as a binding protein through the C-terminal proline-rich region of STAP-2. Expression of STAP-2 increased the interaction between CAP and c-Cbl, suggesting that STAP-2 bridges these proteins and enhances complex formation. CAP/c-Cbl complex is known to regulate GLUT4 translocation in insulin signaling. STAP-2 overexpressed human hepatocyte Hep3B cells showed enhanced GLUT4 translocation after insulin treatment. Elevated levels of Stap2 mRNA have been observed in 3T3-L1 cells and mouse embryonic fibroblasts (MEFs) during adipocyte differentiation. The differentiation of 3T3-L1 cells into adipocytes was highly promoted by retroviral overexpression of STAP-2. In contrast, STAP-2 knockout (KO) MEFs exhibited suppressed adipogenesis. The increase in body weight with high-fat diet feeding was significantly decreased in STAP-2 KO mice compared to WT animals. These data suggest that the expression of STAP-2 correlates with adipogenesis. Thus, STAP-2 is a novel regulatory molecule that controls insulin signal transduction by forming a c-Cbl/STAP-2/CAP ternary complex.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
A novel, multifuntional c-Cbl binding protein in insulin receptor signaling in 3T3-L1 adipocytes.Mol Cell Biol. 1998 Feb;18(2):872-9. doi: 10.1128/MCB.18.2.872. Mol Cell Biol. 1998. PMID: 9447983 Free PMC article.
-
APS facilitates c-Cbl tyrosine phosphorylation and GLUT4 translocation in response to insulin in 3T3-L1 adipocytes.Mol Cell Biol. 2002 Jun;22(11):3599-609. doi: 10.1128/MCB.22.11.3599-3609.2002. Mol Cell Biol. 2002. PMID: 11997497 Free PMC article.
-
The protein content of an adaptor protein, STAP-2 is controlled by E3 ubiquitin ligase Cbl.Biochem Biophys Res Commun. 2009 Jun 26;384(2):187-92. doi: 10.1016/j.bbrc.2009.04.109. Epub 2009 May 3. Biochem Biophys Res Commun. 2009. PMID: 19401194
-
Adaptor protein STAP-2 modulates cellular signaling in immune systems.Biol Pharm Bull. 2014;37(2):185-94. doi: 10.1248/bpb.b13-00421. Biol Pharm Bull. 2014. PMID: 24492713 Review.
-
[Novel adaptor protein, STAP-2 functions as a signal modulator in immune system].Yakugaku Zasshi. 2010 Jun;130(6):769-75. doi: 10.1248/yakushi.130.769. Yakugaku Zasshi. 2010. PMID: 20519854 Review. Japanese.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

