Mucosal tumor vaccination delivering endogenous tumor antigens protects against pulmonary breast cancer metastases
- PMID: 38458636
- PMCID: PMC10921546
- DOI: 10.1136/jitc-2023-008652
Mucosal tumor vaccination delivering endogenous tumor antigens protects against pulmonary breast cancer metastases
Abstract
Background: Generally, early-stage breast cancer has a good prognosis. However, if it spreads systemically, especially with pulmonary involvement, prospects worsen dramatically. Importantly, tumor-infiltrating T cells contribute to tumor control, particularly intratumoral T cells with a tissue-resident memory phenotype are associated with an improved clinical outcome.
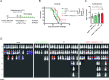
Methods: Here, we use an adenoviral vector vaccine encoding endogenous tumor-associated antigens adjuvanted with interleukin-1β to induce tumor-specific tissue-resident memory T cells (TRM) in the lung for the prevention and treatment of pulmonary metastases in the murine 4T1 breast cancer model.
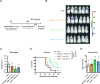
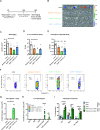
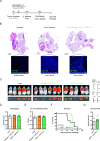
Results: The mucosal delivery of the vaccine was highly efficient in establishing tumor-specific TRM in the lung. Concomitantly, a single mucosal vaccination reduced the growth of pulmonary metastases and improved the survival in a prophylactic treatment. Vaccine-induced TRM contributed to these protective effects. In a therapeutic setting, the vaccination induced a pronounced T cell infiltration into metastases but resulted in only a minor restriction of the disease progression. However, in combination with stereotactic radiotherapy, the vaccine increased the survival time and rate of tumor-bearing mice.
Conclusion: In summary, our study demonstrates that mucosal vaccination is a promising strategy to harness the power of antitumor TRM and its potential combination with state-of-the-art treatments.
Keywords: adjuvant; breast cancer; memory; radiotherapy/radioimmunotherapy; vaccine.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



Similar articles
-
CXCR6 deficiency impairs cancer vaccine efficacy and CD8+ resident memory T-cell recruitment in head and neck and lung tumors.J Immunother Cancer. 2021 Mar;9(3):e001948. doi: 10.1136/jitc-2020-001948. J Immunother Cancer. 2021. PMID: 33692218 Free PMC article.
-
Mucosal BCG Vaccination Induces Protective Lung-Resident Memory T Cell Populations against Tuberculosis.mBio. 2016 Nov 22;7(6):e01686-16. doi: 10.1128/mBio.01686-16. mBio. 2016. PMID: 27879332 Free PMC article.
-
Induction of resident memory T cells enhances the efficacy of cancer vaccine.Nat Commun. 2017 May 24;8:15221. doi: 10.1038/ncomms15221. Nat Commun. 2017. PMID: 28537262 Free PMC article.
-
Tumor Resident Memory T Cells: New Players in Immune Surveillance and Therapy.Front Immunol. 2018 Sep 11;9:2076. doi: 10.3389/fimmu.2018.02076. eCollection 2018. Front Immunol. 2018. PMID: 30258445 Free PMC article. Review.
-
Targeting Resident Memory T Cells for Cancer Immunotherapy.Front Immunol. 2018 Jul 27;9:1722. doi: 10.3389/fimmu.2018.01722. eCollection 2018. Front Immunol. 2018. PMID: 30100906 Free PMC article. Review.
Cited by
-
Mannan-Decorated Lipid Calcium Phosphate Nanoparticle Vaccine Increased the Antitumor Immune Response by Modulating the Tumor Microenvironment.J Funct Biomater. 2024 Aug 16;15(8):229. doi: 10.3390/jfb15080229. J Funct Biomater. 2024. PMID: 39194667 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
