Biomaterial engineering strategies for B cell immunity modulations
- PMID: 38456305
- PMCID: PMC11019864
- DOI: 10.1039/d3bm01841e
Biomaterial engineering strategies for B cell immunity modulations
Abstract
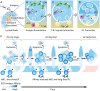
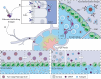
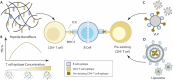
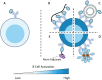
B cell immunity has a penetrating effect on human health and diseases. Therapeutics aiming to modulate B cell immunity have achieved remarkable success in combating infections, autoimmunity, and malignancies. However, current treatments still face significant limitations in generating effective long-lasting therapeutic B cell responses for many conditions. As the understanding of B cell biology has deepened in recent years, clearer regulation networks for B cell differentiation and antibody production have emerged, presenting opportunities to overcome current difficulties and realize the full therapeutic potential of B cell immunity. Biomaterial platforms have been developed to leverage these emerging concepts to augment therapeutic humoral immunity by facilitating immunogenic reagent trafficking, regulating T cell responses, and modulating the immune microenvironment. Moreover, biomaterial engineering tools have also advanced our understanding of B cell biology, further expediting the development of novel therapeutics. In this review, we will introduce the general concept of B cell immunobiology and highlight key biomaterial engineering strategies in the areas including B cell targeted antigen delivery, sustained B cell antigen delivery, antigen engineering, T cell help optimization, and B cell suppression. We will also discuss our perspective on future biomaterial engineering opportunities to leverage humoral immunity for therapeutics.
Conflict of interest statement
The authors have no conflicts of interest.
Figures






Similar articles
-
Ex vivo synthetic immune tissues with T cell signals for differentiating antigen-specific, high affinity germinal center B cells.Biomaterials. 2019 Apr;198:27-36. doi: 10.1016/j.biomaterials.2018.06.034. Epub 2018 Jun 26. Biomaterials. 2019. PMID: 30041943 Free PMC article.
-
Biomaterials as Local Niches for Immunomodulation.Acc Chem Res. 2020 Sep 15;53(9):1749-1760. doi: 10.1021/acs.accounts.0c00341. Epub 2020 Aug 13. Acc Chem Res. 2020. PMID: 32786230
-
Biomaterials for chimeric antigen receptor T cell engineering.Acta Biomater. 2023 Aug;166:1-13. doi: 10.1016/j.actbio.2023.04.043. Epub 2023 May 2. Acta Biomater. 2023. PMID: 37137403 Review.
-
Prospects and challenges for use of CAR T cell therapies in solid tumors.Expert Opin Biol Ther. 2020 May;20(5):503-516. doi: 10.1080/14712598.2020.1738378. Epub 2020 Mar 12. Expert Opin Biol Ther. 2020. PMID: 32125191 Review.
-
Mechanical Immunoengineering of T cells for Therapeutic Applications.Acc Chem Res. 2020 Dec 15;53(12):2777-2790. doi: 10.1021/acs.accounts.0c00486. Epub 2020 Dec 1. Acc Chem Res. 2020. PMID: 33258577 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

