Integrin restriction by miR-34 protects germline progenitors from cell death during aging
- PMID: 38450871
- PMCID: PMC11166360
- DOI: 10.1111/acel.14131
Integrin restriction by miR-34 protects germline progenitors from cell death during aging
Abstract
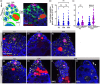
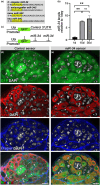
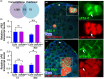
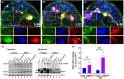
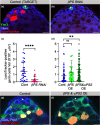
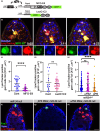
During aging, regenerative tissues must dynamically balance the two opposing processes of proliferation and cell death. While many microRNAs are differentially expressed during aging, their roles as dynamic regulators of tissue regeneration have yet to be described. We show that in the highly regenerative Drosophila testis, miR-34 levels are significantly elevated during aging. miR-34 modulates germ cell death and protects the progenitor germ cells from accelerated aging. However, miR-34 is not expressed in the progenitors themselves but rather in neighboring cyst cells that kill the progenitors. Transcriptomics followed by functional analysis revealed that during aging, miR-34 modifies integrin signaling by limiting the levels of the heterodimeric integrin receptor αPS2 and βPS subunits. In addition, we found that in cyst cells, this heterodimer is essential for inducing phagoptosis and degradation of the progenitor germ cells. Together, these data suggest that the miR-34-integrin signaling axis acts as a sensor of progenitor germ cell death to extend progenitor functionality during aging.
Keywords: drosophila; miR‐34; aging; integrin; micro‐RNAs; phagoptosis; spermatogenesis.
© 2024 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The phagocytic cyst cells in Drosophila testis eliminate germ cell progenitors via phagoptosis.Sci Adv. 2022 Jun 17;8(24):eabm4937. doi: 10.1126/sciadv.abm4937. Epub 2022 Jun 17. Sci Adv. 2022. PMID: 35714186 Free PMC article.
-
The Dlg Module and Clathrin-Mediated Endocytosis Regulate EGFR Signaling and Cyst Cell-Germline Coordination in the Drosophila Testis.Stem Cell Reports. 2019 May 14;12(5):1024-1040. doi: 10.1016/j.stemcr.2019.03.008. Epub 2019 Apr 18. Stem Cell Reports. 2019. PMID: 31006632 Free PMC article.
-
Competitiveness for the niche and mutual dependence of the germline and somatic stem cells in the Drosophila testis are regulated by the JAK/STAT signaling.J Cell Physiol. 2010 May;223(2):500-10. doi: 10.1002/jcp.22073. J Cell Physiol. 2010. PMID: 20143337 Free PMC article.
-
Nutritional regulation of stem and progenitor cells in Drosophila.Development. 2013 Dec;140(23):4647-56. doi: 10.1242/dev.079087. Development. 2013. PMID: 24255094 Free PMC article. Review.
-
MicroRNA function in Drosophila melanogaster.Semin Cell Dev Biol. 2017 May;65:29-37. doi: 10.1016/j.semcdb.2016.03.015. Epub 2016 Mar 18. Semin Cell Dev Biol. 2017. PMID: 27000418 Free PMC article. Review.
References
-
- Alenzi, F. Q. , Alenazi, B. Q. , Ahmad, S. Y. , Salem, M. L. , Al‐Jabri, A. A. , & Wyse, R. K. (2009). The haemopoietic stem cell: Between apoptosis and self renewal. The Yale Journal of Biology and Medicine, 82(1), 7–18. https://www.ncbi.nlm.nih.gov/pubmed/19325941 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

