Echinatin mitigates sevoflurane-induced neurotoxicity through regulation of ferroptosis and iron homeostasis
- PMID: 38446592
- PMCID: PMC10968708
- DOI: 10.18632/aging.205622
Echinatin mitigates sevoflurane-induced neurotoxicity through regulation of ferroptosis and iron homeostasis
Abstract
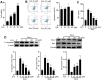
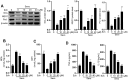
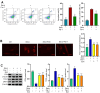
Surgery and anesthesia are vital medical interventions, but concerns over their potential cognitive side effects, particularly with the use of inhalational anesthetics like sevoflurane, have surfaced. This study delves into the neuroprotective potential of Echinatin against sevoflurane-induced neurotoxicity and the underlying mechanisms. Echinatin, a natural compound, has exhibited anti-inflammatory, antioxidant, and anticancer properties. Sevoflurane, while a popular anesthetic, is associated with perioperative neurocognitive disorders (PND) and neurotoxicity. Our investigation began with cellular models, where Echinatin demonstrated a significant reduction in sevoflurane-induced apoptosis. Mechanistically, we identified ferroptosis, a novel form of programmed cell death characterized by iron accumulation and lipid peroxidation, as a key player in sevoflurane-induced neuronal injury. Echinatin notably suppressed ferroptosis in sevoflurane-exposed cells, suggesting a pivotal role in neuroprotection. Expanding our research to a murine model, we observed perturbations in iron homeostasis, inflammatory cytokines, and antioxidants due to sevoflurane exposure. Echinatin treatment effectively restored iron balance, mitigated inflammation, and preserved antioxidant levels in vivo. Behavioral assessments using the Morris water maze further confirmed Echinatin's neuroprotective potential, as it ameliorated sevoflurane-induced spatial learning and memory impairments. In conclusion, our study unveils Echinatin as a promising candidate for mitigating sevoflurane-induced neurotoxicity. Through the regulation of ferroptosis, iron homeostasis, and inflammation, Echinatin demonstrates significant neuroprotection both in vitro and in vivo. These findings illuminate the potential for Echinatin to enhance the safety of surgical procedures involving sevoflurane anesthesia, minimizing the risk of cognitive deficits and neurotoxicity.
Keywords: ALOX12; Echinatin; ferroptosis; neurotoxicity; sevoflurane.
Conflict of interest statement
Figures

Similar articles
-
Echinatin mitigates sevoflurane-induced hippocampal neurotoxicity and cognitive deficits through mitigation of iron overload and oxidative stress.Pharm Biol. 2022 Dec;60(1):1915-1924. doi: 10.1080/13880209.2022.2123941. Pharm Biol. 2022. PMID: 36205592 Free PMC article.
-
Overexpression of Nfs1 Cysteine Desulphurase Relieves Sevoflurane-Induced Neurotoxicity and Cognitive Dysfunction in Neonatal Mice Via Suppressing Oxidative Stress and Ferroptosis.J Biochem Mol Toxicol. 2024 Nov;38(11):e70051. doi: 10.1002/jbt.70051. J Biochem Mol Toxicol. 2024. PMID: 39488760
-
Dysregulation of iron homeostasis and ferroptosis in sevoflurane and isoflurane associated perioperative neurocognitive disorders.CNS Neurosci Ther. 2024 Feb;30(2):e14553. doi: 10.1111/cns.14553. CNS Neurosci Ther. 2024. PMID: 38334231 Free PMC article. Review.
-
Iron overload contributes to general anaesthesia-induced neurotoxicity and cognitive deficits.J Neuroinflammation. 2020 Apr 11;17(1):110. doi: 10.1186/s12974-020-01777-6. J Neuroinflammation. 2020. PMID: 32276637 Free PMC article.
-
Anesthesia and the Developing Brain: A Review of Sevoflurane-induced Neurotoxicity in Pediatric Populations.Clin Ther. 2021 Apr;43(4):762-778. doi: 10.1016/j.clinthera.2021.01.024. Epub 2021 Mar 3. Clin Ther. 2021. PMID: 33674065 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

