Synthesis and evaluation of fluorine-18 labelled tetrazines as pre-targeting imaging agents for PET
- PMID: 38446356
- PMCID: PMC10917718
- DOI: 10.1186/s41181-024-00250-6
Synthesis and evaluation of fluorine-18 labelled tetrazines as pre-targeting imaging agents for PET
Abstract
Background: The brain is a challenging target for antibody-based positron emission tomography (immunoPET) imaging due to the restricted access of antibody-based ligands through the blood-brain barrier (BBB). To overcome this physiological obstacle, we have previously developed bispecific antibody ligands that pass through the BBB via receptor-mediated transcytosis. While these radiolabelled ligands have high affinity and specificity, their long residence time in the blood and brain, typical for large molecules, poses another challenge for PET imaging. A viable solution could be a two-step pre-targeting approach which involves the administration of a tagged antibody that accumulates at the target site in the brain and then clears from the blood, followed by administration of a small radiolabelled molecule with fast kinetics. This radiolabelled molecule can couple to the tagged antibody and thereby make the antibody localisation visible by PET imaging. The in vivo linkage can be achieved by using the inverse electron demand Diels-Alder reaction (IEDDA), with trans-cyclooctene (TCO) and tetrazine groups participating as reactants. In this study, two novel 18F-labelled tetrazines were synthesized and evaluated for their potential use as pre-targeting imaging agents, i.e., for their ability to rapidly enter the brain and, if unbound, to be efficiently cleared with minimal background retention.
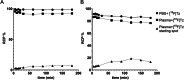
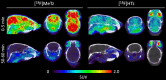
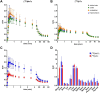
Results: The two compounds, a methyl tetrazine [18F]MeTz and an H-tetrazine [18F]HTz were radiolabelled using a two-step procedure via [18F]F-Py-TFP synthesized on solid support followed by amidation with amine-bearing tetrazines, resulting in radiochemical yields of 24% and 22%, respectively, and a radiochemical purity of > 96%. In vivo PET imaging was performed to assess their suitability for in vivo pre-targeting. Time-activity curves from PET-scans showed [18F]MeTz to be the more pharmacokinetically suitable agent, given its fast and homogenous distribution in the brain and rapid clearance. However, in terms of rection kinetics, H-tetrazines are advantageous, exhibiting faster reaction rates in IEDDA reactions with dienophiles like trans-cyclooctenes, making [18F]HTz potentially more beneficial for pre-targeting applications.
Conclusion: This study demonstrates a significant potential of [18F]MeTz and [18F]HTz as agents for pre-targeted PET brain imaging due to their efficient brain uptake, swift clearance and appropriate chemical stability.
Keywords: Alzheimer’s disease; Bioorthogonal; Fluorine-18; IEDDA; Inverse electron demand Diels–Alder reaction; PET; Pre-targeting; TCO; Tetrazine; Trans-cyclooctene.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
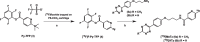
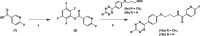
Similar articles
-
Evaluation of the inverse electron demand Diels-Alder reaction in rats using a scandium-44-labelled tetrazine for pretargeted PET imaging.EJNMMI Res. 2019 May 28;9(1):49. doi: 10.1186/s13550-019-0520-y. EJNMMI Res. 2019. PMID: 31140047 Free PMC article.
-
Improved stability of a novel fluorine-18 labeled TCO analogue for pretargeted PET imaging.Nucl Med Biol. 2019 Sep-Oct;76-77:36-42. doi: 10.1016/j.nucmedbio.2019.11.001. Epub 2019 Nov 5. Nucl Med Biol. 2019. PMID: 31707309
-
Micro-flow photosynthesis of new dienophiles for inverse-electron-demand Diels-Alder reactions. Potential applications for pretargeted in vivo PET imaging.Chem Sci. 2017 Feb 1;8(2):1251-1258. doi: 10.1039/c6sc02933g. Epub 2016 Oct 7. Chem Sci. 2017. PMID: 28451267 Free PMC article.
-
The inverse electron demand Diels-Alder cycloaddition with carbon-11 and fluorine-18: A gateway to pretargeted imaging across the blood-brain barrier.J Labelled Comp Radiopharm. 2023 Jul;66(9):249-268. doi: 10.1002/jlcr.4029. Epub 2023 May 18. J Labelled Comp Radiopharm. 2023. PMID: 37147795 Review.
-
Tetrazine-trans-cyclooctene ligation: Unveiling the chemistry and applications within the human body.Bioorg Chem. 2024 Sep;150:107573. doi: 10.1016/j.bioorg.2024.107573. Epub 2024 Jun 18. Bioorg Chem. 2024. PMID: 38905885 Review.
References
-
- Bredack C, Edelmann MR, Borroni E, Gobbi LC, Honer M. Antibody-based in vivo imaging of central nervous system targets—evaluation of a pretargeting approach utilizing a TCO-conjugated brain shuttle antibody and radiolabeled tetrazines. Pharmaceuticals. 2022;15(12):1445. doi: 10.3390/ph15121445. - DOI - PMC - PubMed
-
- Chang C-Y, Chen J-Y, Ke D-S. Essential fatty acids and human brain. Acta Neurol Taiwan. 2009;18:231–241. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
