3-styrylcoumarin scaffold-based derivatives as a new approach for leishmaniasis intervention: biological and molecular modeling studies
- PMID: 38440753
- PMCID: PMC10908709
- DOI: 10.1007/s12639-023-01639-x
3-styrylcoumarin scaffold-based derivatives as a new approach for leishmaniasis intervention: biological and molecular modeling studies
Abstract
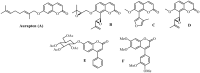
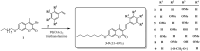
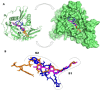
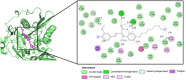
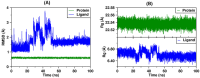
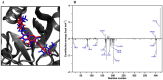
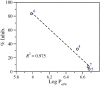
Seven 3-styrylcoumarins were tested for antileishmanial activity against Leishmania (Viannia) panamensis amastigotes. Cytotoxic activity was also evaluated against mammalian U-937 cells. The 3-methoxy-4-hydroxy coumarin derivative 6 was the most active with an IC50 of 40.5 µM, and did not reveal any conspicuous toxicity toward mammalian U-937 cells. Therefore, it may have potential to be considered as candidate for antileishmanial drug development. Further, among several druggable Leishmania targets, molecular docking studies revealed that compound 6 had docking preference by the N-myristoyltransferase (Lp-NMT) of Leishmania panamensis, showing a higher docking score of - 10.1 kcal mol-1 than positive controls and making this protein as a presumably druggable target for this compound. On the other hand, molecular dynamics simulations affirm the docking hypothesis, showing a conformational stability of the 6/Lp-NMT complex throughout 100 ns simulation. Moreover, the molecular mechanics/Poisson-Boltzmann surface area method also support the docking findings, revealing a total free energy of binding of - 47.26 ± 0.08 kcal mol-1, and identifying through energy decomposition analysis that those key aminoacids are contributing strongly to ligand binding. Finally, an optimal pharmacokinetic profile was also estimated for 6. Altogether, coumarin 6 could be addressed as starting point for further pharmacological studies concerning the therapeutic leishmaniasis intervention.
Keywords: 3-styrylcoumarins; Docking studies; In-silico pharmacokinetic evaluation; Leishmaniasis; Molecular modeling studies.
© The Author(s) 2024.
Conflict of interest statement
Conflict of interestThe authors declare no conflict of interest, financial or otherwise.
Figures

Similar articles
-
Homology Modeling, de Novo Design of Ligands, and Molecular Docking Identify Potential Inhibitors of Leishmania donovani 24-Sterol Methyltransferase.Front Cell Infect Microbiol. 2022 Jun 2;12:859981. doi: 10.3389/fcimb.2022.859981. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35719359 Free PMC article.
-
Synthesis, antileishmanial activity and molecular modeling of new 1-aryl/alkyl-3-benzoyl/cyclopropanoyl thiourea derivatives.Mol Divers. 2023 Aug;27(4):1531-1545. doi: 10.1007/s11030-022-10508-3. Epub 2022 Aug 24. Mol Divers. 2023. PMID: 36001225
-
Synthesis, Molecular Docking, Molecular Dynamics Studies, and Biological Evaluation of 4H-Chromone-1,2,3,4-tetrahydropyrimidine-5-carboxylate Derivatives as Potential Antileukemic Agents.J Chem Inf Model. 2017 Jun 26;57(6):1246-1257. doi: 10.1021/acs.jcim.6b00138. Epub 2017 May 25. J Chem Inf Model. 2017. PMID: 28524659
-
Molecular Targets for Chalcones in Antileishmanial Drug Discovery.Mini Rev Med Chem. 2023;23(14):1414-1434. doi: 10.2174/1389557523666230127125058. Mini Rev Med Chem. 2023. PMID: 36705240 Review.
-
Unveiling the Targets Involved in the Quest of Antileishmanial Leads Using In silico Methods.Curr Drug Targets. 2020;21(7):681-712. doi: 10.2174/1389450121666200128112948. Curr Drug Targets. 2020. PMID: 32003668 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
