Therapeutic role of miR-19a/b protection from influenza virus infection in patients with coronary heart disease
- PMID: 38435118
- PMCID: PMC10907223
- DOI: 10.1016/j.omtn.2024.102149
Therapeutic role of miR-19a/b protection from influenza virus infection in patients with coronary heart disease
Abstract
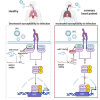
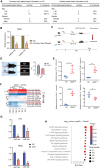
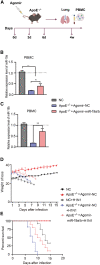
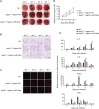
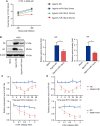
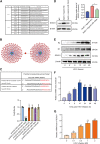
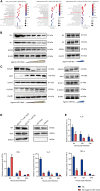
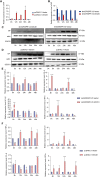
Patients with pre-existing medical conditions are at a heightened risk of contracting severe acute respiratory syndrome (SARS), SARS-CoV-2, and influenza viruses, which can result in more severe disease progression and increased mortality rates. Nevertheless, the molecular mechanism behind this phenomenon remained largely unidentified. Here, we found that microRNA-19a/b (miR-19a/b), which is a constituent of the miR-17-92 cluster, exhibits reduced expression levels in patients with coronary heart disease in comparison to healthy individuals. The downregulation of miR-19a/b has been observed to facilitate the replication of influenza A virus (IAV). miR-19a/b can effectively inhibit IAV replication by targeting and reducing the expression of SOCS1, as observed in cell-based and coronary heart disease mouse models. This mechanism leads to the alleviation of the inhibitory effect of SOCS1 on the interferon (IFN)/JAK/STAT signaling pathway. The results indicate that the IAV employs a unique approach to inhibit the host's type I IFN-mediated antiviral immune responses by decreasing miR-19a/b. These findings provide additional insights into the underlying mechanisms of susceptibility to flu in patients with coronary heart disease. miR-19a/b can be considered as a preventative/therapy strategy for patients with coronary heart disease against influenza virus infection.
Keywords: MT: non-coding RNAs; SOCS1; coronary heart disease; influenza virus; innate immunity; miR-19a/19b.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Human microRNA-30 inhibits influenza virus infection by suppressing the expression of SOCS1, SOCS3, and NEDD4.Cell Microbiol. 2020 May;22(5):e13150. doi: 10.1111/cmi.13150. Epub 2019 Dec 26. Cell Microbiol. 2020. PMID: 31876380 Free PMC article.
-
miR-19a: an effective regulator of SOCS3 and enhancer of JAK-STAT signalling.PLoS One. 2013 Jul 22;8(7):e69090. doi: 10.1371/journal.pone.0069090. Print 2013. PLoS One. 2013. PMID: 23894411 Free PMC article.
-
The Coronavirus Transmissible Gastroenteritis Virus Evades the Type I Interferon Response through IRE1α-Mediated Manipulation of the MicroRNA miR-30a-5p/SOCS1/3 Axis.J Virol. 2018 Oct 29;92(22):e00728-18. doi: 10.1128/JVI.00728-18. Print 2018 Nov 15. J Virol. 2018. PMID: 30185587 Free PMC article.
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
Modulation of Innate Immune Responses by the Influenza A NS1 and PA-X Proteins.Viruses. 2018 Dec 12;10(12):708. doi: 10.3390/v10120708. Viruses. 2018. PMID: 30545063 Free PMC article. Review.
References
-
- Si L., Xu H., Zhou X., Zhang Z., Tian Z., Wang Y., Wu Y., Zhang B., Niu Z., Zhang C., et al. Generation of influenza A viruses as live but replication-incompetent virus vaccines. Science. 2016;354:1170–1173. - PubMed
-
- Pan Y., Cui S., Sun Y., Zhang X., Ma C., Shi W., Peng X., Lu G., Zhang D., Liu Y., et al. Human infection with H9N2 avian influenza in northern China. Clin. Microbiol. Infect. 2018;24:321–323. - PubMed
-
- Tumpey T.M., Basler C.F., Aguilar P.V., Zeng H., Solórzano A., Swayne D.E., Cox N.J., Katz J.M., Taubenberger J.K., Palese P., García-Sastre A. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310:77–80. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

