Fat infiltration in skeletal muscle: Influential triggers and regulatory mechanism
- PMID: 38433917
- PMCID: PMC10907799
- DOI: 10.1016/j.isci.2024.109221
Fat infiltration in skeletal muscle: Influential triggers and regulatory mechanism
Abstract
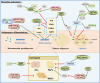
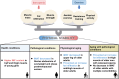
Fat infiltration in skeletal muscle (also known as myosteatosis) is now recognized as a distinct disease from sarcopenia and is directly related to declining muscle capacity. Hence, understanding the origins and regulatory mechanisms of fat infiltration is vital for maintaining skeletal muscle development and improving human health. In this article, we summarized the triggering factors such as aging, metabolic diseases and metabolic syndromes, nonmetabolic diseases, and muscle injury that all induce fat infiltration in skeletal muscle. We discussed recent advances on the cellular origins of fat infiltration and found several cell types including myogenic cells and non-myogenic cells that contribute to myosteatosis. Furthermore, we reviewed the molecular regulatory mechanism, detection methods, and intervention strategies of fat infiltration in skeletal muscle. Based on the current findings, our review will provide new insight into regulating function and lipid metabolism of skeletal muscle and treating muscle-related diseases.
Keywords: Health sciences; Human metabolism; Physiology.
© 2024 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Myosteatosis in the Context of Skeletal Muscle Function Deficit: An Interdisciplinary Workshop at the National Institute on Aging.Front Physiol. 2020 Aug 7;11:963. doi: 10.3389/fphys.2020.00963. eCollection 2020. Front Physiol. 2020. PMID: 32903666 Free PMC article. Review.
-
Factors inducing transdifferentiation of myoblasts into adipocytes.J Cell Physiol. 2021 Apr;236(4):2276-2289. doi: 10.1002/jcp.30074. Epub 2020 Sep 28. J Cell Physiol. 2021. PMID: 32989814 Review.
-
Updated systematic review and meta-analysis on diagnostic issues and the prognostic impact of myosteatosis: A new paradigm beyond sarcopenia.Ageing Res Rev. 2021 Sep;70:101398. doi: 10.1016/j.arr.2021.101398. Epub 2021 Jun 29. Ageing Res Rev. 2021. PMID: 34214642 Review.
-
Psoriasis Is Associated With Myosteatosis but Not Sarcopenia: A Case-Control Study.Front Med (Lausanne). 2021 Oct 15;8:754932. doi: 10.3389/fmed.2021.754932. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34722590 Free PMC article.
-
A dynamic association between myosteatosis and liver stiffness: Results from a prospective interventional study in obese patients.JHEP Rep. 2021 Jun 15;3(4):100323. doi: 10.1016/j.jhepr.2021.100323. eCollection 2021 Aug. JHEP Rep. 2021. PMID: 34355155 Free PMC article.
Cited by
-
Analysis of body composition, functionality and muscle-specific strength of older women with obesity, sarcopenia and sarcopenic obesity: a cross-sectional study.Sci Rep. 2024 Oct 22;14(1):24802. doi: 10.1038/s41598-024-76417-7. Sci Rep. 2024. PMID: 39438648 Free PMC article.
-
Diagnostic Accuracy of Ultrasound Imaging and Shear Wave Elastography to Discriminate Patients with Chronic Neck Pain from Asymptomatic Individuals.Healthcare (Basel). 2024 Oct 5;12(19):1987. doi: 10.3390/healthcare12191987. Healthcare (Basel). 2024. PMID: 39408167 Free PMC article.
-
Decreased skeletal muscle intramyocellular lipid droplet-mitochondrial contact contributes to myosteatosis in cancer cachexia.Am J Physiol Cell Physiol. 2024 Sep 1;327(3):C684-C697. doi: 10.1152/ajpcell.00345.2024. Epub 2024 Jul 16. Am J Physiol Cell Physiol. 2024. PMID: 39010842
-
The effects of myosteatosis on skeletal muscle function in older adults.Physiol Rep. 2024 May;12(9):e16042. doi: 10.14814/phy2.16042. Physiol Rep. 2024. PMID: 38705872 Free PMC article. Review.
-
Integrative cross-species analysis reveals conserved and unique signatures in fatty skeletal muscles.Sci Data. 2024 Mar 12;11(1):290. doi: 10.1038/s41597-024-03114-5. Sci Data. 2024. PMID: 38472209 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

