A1-reprogrammed mesenchymal stromal cells prime potent antitumoral responses
- PMID: 38433914
- PMCID: PMC10907831
- DOI: 10.1016/j.isci.2024.109248
A1-reprogrammed mesenchymal stromal cells prime potent antitumoral responses
Abstract
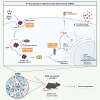
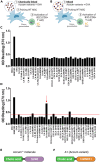
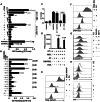
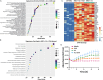
Mesenchymal stromal cells (MSCs) have been modified via genetic or pharmacological engineering into potent antigen-presenting cells-like capable of priming responding CD8 T cells. In this study, our screening of a variant library of Accum molecule revealed a molecule (A1) capable of eliciting antigen cross-presentation properties in MSCs. A1-reprogrammed MSCs (ARM) exhibited improved soluble antigen uptake and processing. Our comprehensive analysis, encompassing cross-presentation assays and molecular profiling, among other cellular investigations, elucidated A1's impact on endosomal escape, reactive oxygen species production, and cytokine secretion. By evaluating ARM-based cellular vaccine in mouse models of lymphoma and melanoma, we observe significant therapeutic potency, particularly in allogeneic setting and in combination with anti-PD-1 immune checkpoint inhibitor. Overall, this study introduces a strong target for developing an antigen-adaptable vaccination platform, capable of synergizing with immune checkpoint blockers to trigger tumor regression, supporting further investigation of ARMs as an effective and versatile anti-cancer vaccine.
Keywords: Cancer; Classification Description; Immunology; Pharmaceutical engineering.
© 2024 The Author(s).
Conflict of interest statement
Daniela Stanga and Sebastien Plouffe are employees of Defense Therapeutics and declare competing financial interests. Moutih Rafei is part of Defense Therapeutics Board.
Figures

Similar articles
-
UM171A-induced ROS promote antigen cross-presentation of immunogenic peptides by bone marrow-derived mesenchymal stromal cells.Stem Cell Res Ther. 2022 Jan 10;13(1):16. doi: 10.1186/s13287-021-02693-z. Stem Cell Res Ther. 2022. PMID: 35012668 Free PMC article.
-
Local delivery of accutox® synergises with immune-checkpoint inhibitors at disrupting tumor growth.J Transl Med. 2024 Jun 3;22(1):532. doi: 10.1186/s12967-024-05340-2. J Transl Med. 2024. PMID: 38831284 Free PMC article.
-
Engineering immunoproteasome-expressing mesenchymal stromal cells: A potent cellular vaccine for lymphoma and melanoma in mice.Cell Rep Med. 2021 Dec 21;2(12):100455. doi: 10.1016/j.xcrm.2021.100455. eCollection 2021 Dec 21. Cell Rep Med. 2021. PMID: 35028603 Free PMC article.
-
Different priming strategies improve distinct therapeutic capabilities of mesenchymal stromal/stem cells: Potential implications for their clinical use.World J Stem Cells. 2023 May 26;15(5):400-420. doi: 10.4252/wjsc.v15.i5.400. World J Stem Cells. 2023. PMID: 37342218 Free PMC article. Review.
-
Mesenchymal stromal cells for bone sarcoma treatment: Roadmap to clinical practice.J Bone Oncol. 2019 Mar 19;16:100231. doi: 10.1016/j.jbo.2019.100231. eCollection 2019 Jun. J Bone Oncol. 2019. PMID: 30956944 Free PMC article. Review.
References
-
- Chiang C.L.L., Kandalaft L.E., Tanyi J., Hagemann A.R., Motz G.T., Svoronos N., Montone K., Mantia-Smaldone G.M., Smith L., Nisenbaum H.L., et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: From bench to bedside. Clin. Cancer Res. 2013;19:4801–4815. doi: 10.1158/1078-0432.CCR-13-1185. - DOI - PMC - PubMed
-
- Liau L.M., Ashkan K., Brem S., Campian J.L., Trusheim J.E., Iwamoto F.M., Tran D.D., Ansstas G., Cobbs C.S., Heth J.A., et al. Association of Autologous Tumor Lysate-Loaded Dendritic Cell Vaccination with Extension of Survival among Patients with Newly Diagnosed and Recurrent Glioblastoma: A Phase 3 Prospective Externally Controlled Cohort Trial. JAMA Oncol. 2023;9:112–121. doi: 10.1001/jamaoncol.2022.5370. - DOI - PMC - PubMed
-
- Dillman R.O., Cornforth A.N., Nistor G.I., McClay E.F., Amatruda T.T., Depriest C. Randomized phase II trial of autologous dendritic cell vaccines versus autologous tumor cell vaccines in metastatic melanoma: 5-year follow up and additional analyses. J. Immunother. Cancer. 2018;6 doi: 10.1186/s40425-018-0330-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

