Exceptionally long-lasting response to dabrafenib plus trametinib treatment in a patient with lung adenocarcinoma harboring the BRAF V600E mutation with high expression of PD-L1: A case report
- PMID: 38429896
- PMCID: PMC11016420
- DOI: 10.1111/1759-7714.15254
Exceptionally long-lasting response to dabrafenib plus trametinib treatment in a patient with lung adenocarcinoma harboring the BRAF V600E mutation with high expression of PD-L1: A case report
Abstract
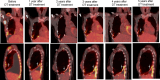
We present a patient with lung adenocarcinoma showing high PD-L1 expression and BRAF V600E mutation, who achieved a remarkable long-term response to the combination therapy of dabrafenib and trametinib (DT treatment) after disease progression on immunotherapy. This case may provide an opportunity for clinicians to consider the order of administration of immunotherapy and molecular targeted therapy for BRAF V600E-positive lung cancer.
Keywords: BRAF V600E; case report; dabrafenib plus trametinib; immunotherapy; non‐small cell lung cancer.
© 2024 The Authors. Thoracic Cancer published by John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare the following financial interests/personal relationships that may be considered as potential competing interests. Dr Inoue reports honoraria for lecture fees from AstraZeneca, Ono, MSD, and Chugai. Dr Kunimasa reports honoraria for lecture fees from AstraZeneca, Chugai Pharma, and Novartis. Dr Tamiya reports receiving grants from Boehringer Ingelheim, Ono, MSD, Eisai, Daiichi Sankyo, Chugai, and Janssen; and personal fees from Boehringer Ingelheim, Ono, MSD, Chugai, AstraZeneca, Taiho, Eli Lilly, Novartis, Asahi Kasei, Bristol‐Myers Squibb, Bayer, Amgen, Kyowa‐Kirin, and Nippon Kayaku. Dr Nishino reports receiving grants from Ono, TAIHO, MSD, AbbVie, DAIICHI SANKYO, Amgen, Eisai, Sanofi, Janssen, Novartis, Pfizer, Eli Lilly, Merck, Takeda, Chugai, and Merus; and personal fees from AstraZeneca, Chugai, Nippon Boehringer Ingerheim, Eli Lilly, Roche, Novartis, Pfizer, Merck, Janssen, Bristol Myers Squibb, and Nippon Kayaku. The remaining authors declare no conflict of interest.
Figures


Similar articles
-
Adverse Event Management in Patients with BRAF V600E-Mutant Non-Small Cell Lung Cancer Treated with Dabrafenib plus Trametinib.Oncologist. 2019 Jul;24(7):963-972. doi: 10.1634/theoncologist.2018-0296. Epub 2018 Dec 31. Oncologist. 2019. PMID: 30598499 Free PMC article.
-
An Acquired NRAS Q61K Mutation in BRAF V600E-Mutant Lung Adenocarcinoma Resistant to Dabrafenib Plus Trametinib.J Thorac Oncol. 2018 Aug;13(8):e131-e133. doi: 10.1016/j.jtho.2018.03.026. Epub 2018 Apr 6. J Thorac Oncol. 2018. PMID: 29631033 Free PMC article. No abstract available.
-
BRAF V600E Mediates Crizotinib Resistance and Responds to Dabrafenib and Trametinib in a ROS1-Rearranged Non-Small Cell Lung Cancer: A Case Report.Oncologist. 2021 Dec;26(12):e2115-e2119. doi: 10.1002/onco.13979. Epub 2021 Nov 9. Oncologist. 2021. PMID: 34516041 Free PMC article.
-
Interstitial lung disease associated with combination therapy of dabrafenib and trametinib in metastatic BRAFV600E-mutated poorly differentiated thyroid cancer: A case report and review of the literature.Int J Clin Pharmacol Ther. 2022 May;60(5):225-231. doi: 10.5414/CP204184. Int J Clin Pharmacol Ther. 2022. PMID: 35072623 Review.
-
Dabrafenib and trametinib for the treatment of non-small cell lung cancer.Expert Rev Anticancer Ther. 2018 Nov;18(11):1063-1068. doi: 10.1080/14737140.2018.1521272. Epub 2018 Sep 13. Expert Rev Anticancer Ther. 2018. PMID: 30198802 Review.
References
-
- US Food and Drug Administration . FDA grants accelerated approval to dabrafenib in combination with trametinib for unresectable or metastatic solid tumors with BRAF V600E mutation, 2023. Accessed 24 November 2023. https://www.fda.gov/drugs/resources‐information‐approved‐drugs/fda‐grant...
-
- Philip B, Martin R, Tobias O. Outcome of first‐line treatment with pembrolizumab according to KRAS/TP53 mutational status for nonsquamous programmed death‐ligand 1‐high (≥50%) NSCLC in the German National Network Genomic Medicine Lung Cancer. J Thorac Oncol. 2023;S1556‐0864(23):02423‐1. 10.1016/j.jtho.2023.12.015 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

