The Alpha-Synuclein Gene (SNCA) is a Genomic Target of Methyl-CpG Binding Protein 2 (MeCP2)-Implications for Parkinson's Disease and Rett Syndrome
- PMID: 38429622
- PMCID: PMC11415397
- DOI: 10.1007/s12035-024-03974-3
The Alpha-Synuclein Gene (SNCA) is a Genomic Target of Methyl-CpG Binding Protein 2 (MeCP2)-Implications for Parkinson's Disease and Rett Syndrome
Abstract
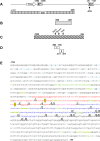
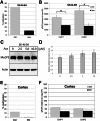
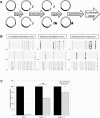
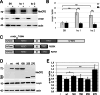
Mounting evidence suggests a prominent role for alpha-synuclein (a-syn) in neuronal cell function. Alterations in the levels of cellular a-syn have been hypothesized to play a critical role in the development of Parkinson's disease (PD); however, mechanisms that control expression of the gene for a-syn (SNCA) in cis and trans as well as turnover of a-syn are not well understood. We analyzed whether methyl-CpG binding protein 2 (MeCP2), a protein that specifically binds methylated DNA, thus regulating transcription, binds at predicted binding sites in intron 1 of the SNCA gene and regulates a-syn protein expression. Chromatin immunoprecipitation (ChIP) and electrophoretic mobility-shift assays (EMSA) were used to confirm binding of MeCP2 to regulatory regions of SNCA. Site-specific methylation and introduction of localized mutations by CRISPR/Cas9 were used to investigate the binding properties of MeCP2 in human SK-N-SH neuroblastoma cells. The significance of MeCP2 for SNCA regulation was further investigated by overexpressing MeCP2 and mutated variants of MeCP2 in MeCP2 knockout cells. We found that methylation-dependent binding of MeCP2 at a restricted region of intron 1 of SNCA had a significant impact on the production of a-syn. A single nucleotide substitution near to CpG1 strongly increased the binding of MeCP2 to intron 1 of SNCA and decreased a-syn protein expression by 60%. In contrast, deletion of a single nucleotide closed to CpG2 led to reduced binding of MeCP2 and significantly increased a-syn levels. In accordance, knockout of MeCP2 in SK-N-SH cells resulted in a significant increase in a-syn production, demonstrating that SNCA is a genomic target for MeCP2 regulation. In addition, the expression of two mutated MeCP2 variants found in Rett syndrome (RTT) showed a loss of their ability to reduce a-syn expression. This study demonstrates that methylation of CpGs and binding of MeCP2 to intron 1 of the SNCA gene plays an important role in the control of a-syn expression. In addition, the changes in SNCA regulation found by expression of MeCP2 variants carrying mutations found in RTT patients may be of importance for the elucidation of a new molecular pathway in RTT, a rare neurological disorder caused by mutations in MECP2.
Keywords: SNCA; Alpha-synuclein; DNA methylation; Epigenetic; Genomic target; Intron; MeCP2; Methyl-CpG binding protein 2; Parkinson’s disease; RTT; Rett syndrome.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
MeCP2 binds to non-CG methylated DNA as neurons mature, influencing transcription and the timing of onset for Rett syndrome.Proc Natl Acad Sci U S A. 2015 Apr 28;112(17):5509-14. doi: 10.1073/pnas.1505909112. Epub 2015 Apr 13. Proc Natl Acad Sci U S A. 2015. PMID: 25870282 Free PMC article.
-
The protocadherins, PCDHB1 and PCDH7, are regulated by MeCP2 in neuronal cells and brain tissues: implication for pathogenesis of Rett syndrome.BMC Neurosci. 2011 Aug 8;12:81. doi: 10.1186/1471-2202-12-81. BMC Neurosci. 2011. PMID: 21824415 Free PMC article.
-
Reciprocal co-regulation of EGR2 and MECP2 is disrupted in Rett syndrome and autism.Hum Mol Genet. 2009 Feb 1;18(3):525-34. doi: 10.1093/hmg/ddn380. Epub 2008 Nov 10. Hum Mol Genet. 2009. PMID: 19000991 Free PMC article.
-
Role of DNA Methyl-CpG-Binding Protein MeCP2 in Rett Syndrome Pathobiology and Mechanism of Disease.Biomolecules. 2021 Jan 8;11(1):75. doi: 10.3390/biom11010075. Biomolecules. 2021. PMID: 33429932 Free PMC article. Review.
-
Regulation mechanism and research progress of MeCP2 in Rett syndrome.Yi Chuan. 2014 Jul;36(7):625-30. doi: 10.3724/SP.J.1005.2014.0625. Yi Chuan. 2014. PMID: 25076025 Review.
Cited by
-
An Overview of Epigenetic Changes in the Parkinson's Disease Brain.Int J Mol Sci. 2024 Jun 3;25(11):6168. doi: 10.3390/ijms25116168. Int J Mol Sci. 2024. PMID: 38892355 Free PMC article. Review.
References
-
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature. 388:839–840 - PubMed
-
- Braak H, Del TK, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211 - PubMed
-
- Yang JO, Kim W-Y, Jeong S-Y, Oh J-H, Jho S, Bhak J, Kim N-S (2009) PDbase: a database of Parkinson’s disease-related genes and genetic variation using substantia nigra ESTs. BMC Genomics 10:S32 http://bioportal.kobic.re.kr/PDbase/;http://bioportal.kobic.re.kr/PDbase/suppl.jsp - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

