Substrate-bound and soluble domains of tenascin-C regulate differentiation, proliferation and migration of neural stem and progenitor cells
- PMID: 38425428
- PMCID: PMC10902920
- DOI: 10.3389/fncel.2024.1357499
Substrate-bound and soluble domains of tenascin-C regulate differentiation, proliferation and migration of neural stem and progenitor cells
Abstract
Introduction: The lack of regenerative capacity of the central nervous system is one of the major challenges nowadays. The knowledge of guidance cues that trigger differentiation, proliferation, and migration of neural stem and progenitor cells is one key element in regenerative medicine. The extracellular matrix protein tenascin-C (Tnc) is a promising candidate to regulate cell fate due to its expression in the developing central nervous system and in the adult neural stem cell niches. Of special interest are the alternatively spliced fibronectin type III (FnIII) domains of Tnc whose combinatorial diversity could theoretically generate up to 64 isoforms in the mouse. A total of 27 isoforms have already been discovered in the developing brain, among others the domain combinations A1D, CD, and A124BCD.
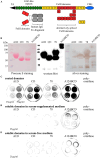
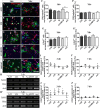
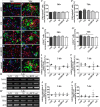
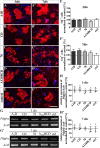
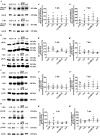
Methods: In the present study, these domains as well as the combination of the constitutively expressed FnIII domains 7 and 8 (78) were expressed in Chinese hamster ovary cells as pseudo-antibodies fused to the Fc-fragment of a human immunoglobulin G antibody. The fusion proteins were presented to primary mouse neural stem/progenitor cells (NSPCs) grown as neurospheres, either as coated culture substrates or as soluble additives in vitro. The influence of the domains on the differentiation, proliferation and migration of NSPCs was analyzed.
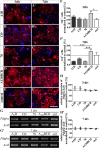
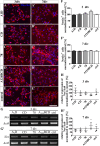
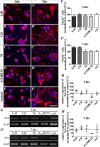
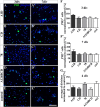
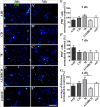
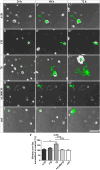
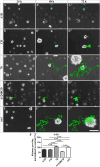
Results: We observed that the domain combination A124BCD promoted the differentiation of neurons and oligodendrocytes, whereas the domain A1D supported astrocyte differentiation. The constitutively expressed domain 78 had a proliferation and migration stimulating impact. Moreover, most effects were seen only in one of the presentation modes but not in both, suggesting different effects of the Tnc domains in two- and three-dimensional cultures.
Discussion: This knowledge about the different effect of the Tnc domains might be used to create artificial three-dimensional environments for cell transplantation. Hydrogels spiked with Tnc-domains might represent a promising tool in regenerative medicine.
Keywords: alternatively spliced fibronectin III domains; dichotomy of coated substrate and soluble additives; differentiation; migration; neural stem/progenitor cells; proliferation; tenascin-C.
Copyright © 2024 Glotzbach and Faissner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Neural stem/progenitor cells express 20 tenascin C isoforms that are differentially regulated by Pax6.J Biol Chem. 2007 Mar 23;282(12):9172-81. doi: 10.1074/jbc.M608067200. Epub 2007 Jan 30. J Biol Chem. 2007. PMID: 17264084
-
Analysis of combinatorial variability reveals selective accumulation of the fibronectin type III domains B and D of tenascin-C in injured brain.Exp Neurol. 2010 Sep;225(1):60-73. doi: 10.1016/j.expneurol.2010.04.019. Epub 2010 May 5. Exp Neurol. 2010. PMID: 20451518
-
Different forms of tenascin-C with tenascin-R regulate neural differentiation in bone marrow-derived human mesenchymal stem cells.Tissue Eng Part A. 2014 Jul;20(13-14):1908-21. doi: 10.1089/ten.TEA.2013.0188. Tissue Eng Part A. 2014. PMID: 24829055 Free PMC article.
-
Expression of tenascin-C and its isoforms in the breast.Cancer Metastasis Rev. 2010 Dec;29(4):595-606. doi: 10.1007/s10555-010-9249-9. Cancer Metastasis Rev. 2010. PMID: 20814719 Review.
-
Tenascins in CNS lesions.Semin Cell Dev Biol. 2019 May;89:118-124. doi: 10.1016/j.semcdb.2018.09.012. Epub 2018 Oct 11. Semin Cell Dev Biol. 2019. PMID: 30287388 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

