Interaction between the SFTSV envelope glycoprotein Gn and STING inhibits the formation of the STING-TBK1 complex and suppresses the NF-κB signaling pathway
- PMID: 38421179
- PMCID: PMC10949458
- DOI: 10.1128/jvi.01815-23
Interaction between the SFTSV envelope glycoprotein Gn and STING inhibits the formation of the STING-TBK1 complex and suppresses the NF-κB signaling pathway
Abstract
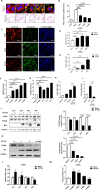
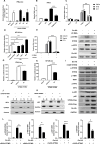
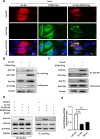
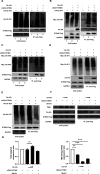
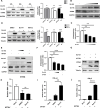
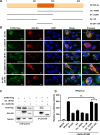
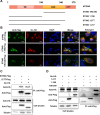
Severe fever with thrombocytopenia syndrome virus (SFTSV) is an emerging tick-borne bunyavirus with high pathogenicity. There has been a gradual increase in the number of reported cases in recent years, with high morbidity and mortality rates. The cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) signaling pathway plays an important role in the innate immune defense activated by viral infection; however, the role of the cGAS-STING signaling pathway during SFTSV infection is still unclear. In this study, we investigated the relationship between SFTSV infection and cGAS-STING signaling. We found that SFTSV infection caused the release of mitochondrial DNA into the cytoplasm and inhibits downstream innate immune signaling pathways by activating the cytoplasmic DNA receptor cGAS. We found that the SFTSV envelope glycoprotein Gn was a potent inhibitor of the cGAS-STING pathway and blocked the nuclear accumulation of interferon regulatory factor 3 and p65 to inhibit downstream innate immune signaling. Gn of SFTSV interacted with STING to inhibit STING dimerization and inhibited K27-ubiquitin modification of STING to disrupt the assembly of the STING-TANK-binding kinase 1 complex and downstream signaling. In addition, Gn was found to be involved in inducing STING degradation, further inhibiting the downstream immune response. In conclusion, this study identified the important role of the glycoprotein Gn in the antiviral innate immune response and revealed a novel mechanism of immune escape for SFTSV. Moreover, this study increases the understanding of the pathogenic mechanism of SFTSV and provides new insights for further treatment of SFTS.
Importance: Severe fever with thrombocytopenia syndrome virus (SFTSV) is a newly discovered virus associated with severe hemorrhagic fever in humans. However, the role of the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) signaling pathway during SFTSV infection is still unclear. We found that SFTSV infection inhibits downstream innate immune signaling pathways by activating the cytoplasmic DNA receptor cGAS. In addition, SFTSV Gn blocked the nuclear accumulation of interferon regulatory factor 3 and p65 to inhibit downstream innate immune signaling. Moreover, we determined that Gn of SFTSV inhibited K27-ubiquitin modification of STING to disrupt the assembly of the STING-TANK-binding kinase 1 complex and downstream signaling. We found that the SFTSV envelope glycoprotein Gn is a potent inhibitor of the cGAS-STING pathway. In conclusion, this study highlights the crucial function of the glycoprotein Gn in the antiviral innate immune response and reveals a new method of immune escape of SFTSV.
Keywords: Gn; cyclic GMP-AMP synthase (cGAS); infection; innate immunity; mitochondrial DNA; severe fever with thrombocytopenia syndrome virus (SFTSV); stimulator of interferon genes (STING).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
SFTSV nucleoprotein mediates DNA sensor cGAS degradation to suppress cGAS-dependent antiviral responses.Microbiol Spectr. 2024 Jun 4;12(6):e0379623. doi: 10.1128/spectrum.03796-23. Epub 2024 May 7. Microbiol Spectr. 2024. PMID: 38712963 Free PMC article.
-
The cGas-Sting Signaling Pathway Is Required for the Innate Immune Response Against Ectromelia Virus.Front Immunol. 2018 Jun 14;9:1297. doi: 10.3389/fimmu.2018.01297. eCollection 2018. Front Immunol. 2018. PMID: 29963044 Free PMC article.
-
African Swine Fever Virus Armenia/07 Virulent Strain Controls Interferon Beta Production through the cGAS-STING Pathway.J Virol. 2019 May 29;93(12):e02298-18. doi: 10.1128/JVI.02298-18. Print 2019 Jun 15. J Virol. 2019. PMID: 30918080 Free PMC article.
-
Role of micronucleus-activated cGAS-STING signaling in antitumor immunity.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024 Jan 26;53(1):25-34. doi: 10.3724/zdxbyxb-2023-0485. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38273467 Free PMC article. Review. Chinese, English.
-
SARS-CoV-2, HIV, and HPV: Convergent evolution of selective regulation of cGAS-STING signaling.J Med Virol. 2023 Jan;95(1):e28220. doi: 10.1002/jmv.28220. Epub 2022 Oct 26. J Med Virol. 2023. PMID: 36229923 Free PMC article. Review.
Cited by
-
Current insights into human pathogenic phenuiviruses and the host immune system.Virulence. 2024 Dec;15(1):2384563. doi: 10.1080/21505594.2024.2384563. Epub 2024 Jul 29. Virulence. 2024. PMID: 39072499 Free PMC article. Review.
References
-
- Kuhn J-O, Adkins S-O, Alioto D-O, Alkhovsky S-O, Amarasinghe G-O, Anthony SJ, Avšič-Županc T-O, Ayllón M-O, Bahl J-O, Balkema-Buschmann A-O, et al. . 2020. 2020 taxonomic update for phylum Negarnaviricota (Riboviria: Orthornavirae), including the large orders Bunyavirales and Mononegavirales - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

