Deciphering Density Fluctuations in the Hydration Water of Brownian Nanoparticles via Upconversion Thermometry
- PMID: 38420927
- PMCID: PMC10926164
- DOI: 10.1021/acs.jpclett.4c00044
Deciphering Density Fluctuations in the Hydration Water of Brownian Nanoparticles via Upconversion Thermometry
Abstract
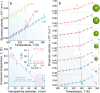
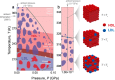
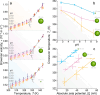
We investigate the intricate relationship among temperature, pH, and Brownian velocity in a range of differently sized upconversion nanoparticles (UCNPs) dispersed in water. These UCNPs, acting as nanorulers, offer insights into assessing the relative proportion of high-density and low-density liquid in the surrounding hydration water. The study reveals a size-dependent reduction in the onset temperature of liquid-water fluctuations, indicating an augmented presence of high-density liquid domains at the nanoparticle surfaces. The observed upper-temperature threshold is consistent with a hypothetical phase diagram of water, validating the two-state model. Moreover, an increase in pH disrupts the organization of water molecules, similar to external pressure effects, allowing simulation of the effects of temperature and pressure on hydrogen bonding networks. The findings underscore the significance of the surface of suspended nanoparticles for understanding high- to low-density liquid fluctuations and water behavior at charged interfaces.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Hydrophobicity of proteins and interfaces: insights from density fluctuations.Annu Rev Chem Biomol Eng. 2011;2:147-71. doi: 10.1146/annurev-chembioeng-061010-114156. Annu Rev Chem Biomol Eng. 2011. PMID: 22432614 Review.
-
Dispersion stability and biocompatibility of four ligand-exchanged NaYF4: Yb, Er upconversion nanoparticles.Acta Biomater. 2020 Jan 15;102:384-393. doi: 10.1016/j.actbio.2019.11.048. Epub 2019 Dec 1. Acta Biomater. 2020. PMID: 31794872
-
Engineering water-tolerant core/shell upconversion nanoparticles for optical temperature sensing.Opt Lett. 2017 Jul 1;42(13):2451-2454. doi: 10.1364/OL.42.002451. Opt Lett. 2017. PMID: 28957257
-
Connecting Non-Gaussian Water Density Fluctuations to the Lengthscale Dependent Crossover in Hydrophobic Hydration.J Phys Chem B. 2022 Oct 6;126(39):7604-7614. doi: 10.1021/acs.jpcb.2c04990. Epub 2022 Sep 26. J Phys Chem B. 2022. PMID: 36154059
-
Hydration and Nanoconfined Water: Insights from Computer Simulations.Subcell Biochem. 2015;71:161-87. doi: 10.1007/978-3-319-19060-0_7. Subcell Biochem. 2015. PMID: 26438265 Review.
References
LinkOut - more resources
Full Text Sources

