Multi-omics reveals deoxycholic acid modulates bile acid metabolism via the gut microbiota to antagonize carbon tetrachloride-induced chronic liver injury
- PMID: 38416424
- PMCID: PMC10903553
- DOI: 10.1080/19490976.2024.2323236
Multi-omics reveals deoxycholic acid modulates bile acid metabolism via the gut microbiota to antagonize carbon tetrachloride-induced chronic liver injury
Abstract
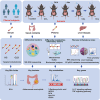
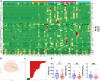
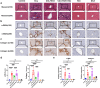
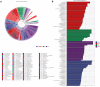
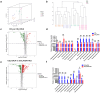
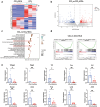
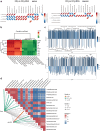
Deoxycholic acid (DCA) serves essential functions in both physiological and pathological liver processes; nevertheless, the relationship among DCA, gut microbiota, and metabolism in chronic liver injury remain insufficiently understood. The primary objective of this study is to elucidate the potential of DCA in ameliorating chronic liver injury and evaluate its regulatory effect on gut microbiota and metabolism via a comprehensive multi-omics approach. Our study found that DCA supplementation caused significant changes in the composition of gut microbiota, which were essential for its antagonistic effect against CCl4-induced chronic liver injury. When gut microbiota was depleted with antibiotics, the observed protective efficacy of DCA against chronic liver injury became noticeably attenuated. Mechanistically, we discovered that DCA regulates the metabolism of bile acids (BAs), including 3-epi DCA, Apo-CA, and its isomers 12-KLCA and 7-KLCA, IHDCA, and DCA, by promoting the growth of A.muciniphila in gut microbiota. This might lead to the inhibition of the IL-17 and TNF inflammatory signaling pathway, thereby effectively countering CCl4-induced chronic liver injury. This study illustrates that the enrichment of A. muciniphila in the gut microbiota, mediated by DCA, enhances the production of secondary bile acids, thereby mitigating chronic liver injury induced by CCl4. The underlying mechanism may involve the inhibition of hepatic IL-17 and TNF signaling pathways. These findings propose a promising approach to alleviate chronic liver injury by modulating both the gut microbiota and bile acids metabolism.
Keywords: Chronic liver injury; deoxycholic acid; gut microbiota; inflammation; metabolism; transcriptome.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



Similar articles
-
Modulation of the Gut Microbiota-farnesoid X Receptor Axis Improves Deoxycholic Acid-induced Intestinal Inflammation in Mice.J Crohns Colitis. 2021 Jul 5;15(7):1197-1210. doi: 10.1093/ecco-jcc/jjab003. J Crohns Colitis. 2021. PMID: 33417675
-
The polysaccharides from Auricularia auricula alleviate non-alcoholic fatty liver disease via modulating gut microbiota and bile acids metabolism.Int J Biol Macromol. 2023 Aug 15;246:125662. doi: 10.1016/j.ijbiomac.2023.125662. Epub 2023 Jul 1. Int J Biol Macromol. 2023. PMID: 37399869
-
Forsythiaside A alleviated carbon tetrachloride-induced liver fibrosis by modulating gut microbiota composition to increase short-chain fatty acids and restoring bile acids metabolism disorder.Biomed Pharmacother. 2022 Jul;151:113185. doi: 10.1016/j.biopha.2022.113185. Epub 2022 May 24. Biomed Pharmacother. 2022. PMID: 35623173
-
Gut microbiota derived bile acid metabolites maintain the homeostasis of gut and systemic immunity.Front Immunol. 2023 May 15;14:1127743. doi: 10.3389/fimmu.2023.1127743. eCollection 2023. Front Immunol. 2023. PMID: 37256134 Free PMC article. Review.
-
The Role of Gut Microbiota-Derived Lithocholic Acid, Deoxycholic Acid and Their Derivatives on the Function and Differentiation of Immune Cells.Microorganisms. 2023 Nov 8;11(11):2730. doi: 10.3390/microorganisms11112730. Microorganisms. 2023. PMID: 38004742 Free PMC article. Review.
Cited by
-
TGR5 deficiency in excitatory neurons ameliorates Alzheimer's pathology by regulating APP processing.Sci Adv. 2024 Jun 28;10(26):eado1855. doi: 10.1126/sciadv.ado1855. Epub 2024 Jun 28. Sci Adv. 2024. PMID: 38941459 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
