MicroRNA-29c-tetrahedral framework nucleic acids: Towards osteogenic differentiation of mesenchymal stem cells and bone regeneration in critical-sized calvarial defects
- PMID: 38414296
- PMCID: PMC11216942
- DOI: 10.1111/cpr.13624
MicroRNA-29c-tetrahedral framework nucleic acids: Towards osteogenic differentiation of mesenchymal stem cells and bone regeneration in critical-sized calvarial defects
Abstract
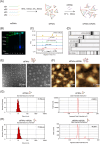
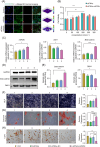
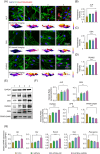
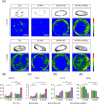
Certain miRNAs, notably miR29c, demonstrate a remarkable capacity to regulate cellular osteogenic differentiation. However, their application in tissue regeneration is hampered by their inherent instability and susceptibility to degradation. In this study, we developed a novel miR29c delivery system utilising tetrahedral framework nucleic acids (tFNAs), aiming to enhance its stability and endocytosis capability, augment the efficacy of miR29c, foster osteogenesis in bone marrow mesenchymal stem cells (BMSCs), and significantly improve the repair of critical-sized bone defects (CSBDs). We confirmed the successful synthesis and biocompatibility of sticky ends-modified tFNAs (stFNAs) and miR29c-modified stFNAs (stFNAs-miR29c) through polyacrylamide gel electrophoresis, microscopy scanning, a cell counting kit-8 assay and so on. The mechanism and osteogenesis effects of stFNAs-miR29c were explored using immunofluorescence staining, western blotting, and reserve transcription quantitative real-time polymerase chain reaction. Additionally, the impact of stFNAs-miR29c on CSBD repair was assessed via micro-CT and histological staining. The nano-carrier, stFNAs-miR29c was successfully synthesised and exhibited exemplary biocompatibility. This nano-nucleic acid material significantly upregulated osteogenic differentiation-related markers in BMSCs. After 2 months, stFNAs-miR29c demonstrated significant bone regeneration and reconstruction in CSBDs. Mechanistically, stFNAs-miR29c enhanced osteogenesis of BMSCs by upregulating the Wnt signalling pathway, contributing to improved bone tissue regeneration. The development of this novel nucleic acid nano-carrier, stFNAs-miR29c, presents a potential new avenue for guided bone regeneration and bone tissue engineering research.
© 2024 The Authors. Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
MiR-26b-5p/TET3 regulates the osteogenic differentiation of human bone mesenchymal stem cells and bone reconstruction in female rats with calvarial defects.Mol Biol Rep. 2024 May 9;51(1):632. doi: 10.1007/s11033-024-09577-4. Mol Biol Rep. 2024. PMID: 38724827
-
A Composite Tissue Engineered Bone Material Consisting of Bone Mesenchymal Stem Cells, Bone Morphogenetic Protein 9 (BMP9) Gene Lentiviral Vector, and P3HB4HB Thermogel (BMSCs-LV-BMP9-P3HB4HB) Repairs Calvarial Skull Defects in Rats by Expression of Osteogenic Factors.Med Sci Monit. 2020 Sep 7;26:e924666. doi: 10.12659/MSM.924666. Med Sci Monit. 2020. PMID: 32894745 Free PMC article.
-
Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration.Cell Prolif. 2019 Sep;52(5):e12669. doi: 10.1111/cpr.12669. Epub 2019 Aug 5. Cell Prolif. 2019. PMID: 31380594 Free PMC article.
-
Repair of critical-sized bone defects with anti-miR-31-expressing bone marrow stromal stem cells and poly(glycerol sebacate) scaffolds.Eur Cell Mater. 2014 Jan 15;27:13-24; discussion 24-5. doi: 10.22203/ecm.v027a02. Eur Cell Mater. 2014. PMID: 24425157
-
Roles for miRNAs in osteogenic differentiation of bone marrow mesenchymal stem cells.Stem Cell Res Ther. 2019 Jun 28;10(1):197. doi: 10.1186/s13287-019-1309-7. Stem Cell Res Ther. 2019. PMID: 31253175 Free PMC article. Review.
References
-
- Li L, Lu H, Zhao Y, et al. Functionalized cell‐free scaffolds for bone defect repair inspired by self‐healing of bone fractures: a review and new perspectives. Mater Sci Eng C. 2019;98:1241‐1251. - PubMed
MeSH terms
Substances
Grants and funding
- 2019YFA0110600/National Key Research and Development Program of China
- 82322015/National Natural Science Foundation of China
- 82171006/National Natural Science Foundation of China
- 81970986/National Natural Science Foundation of China
- 2022JDTD0021/Sichuan Province Youth Science and Technology Innovation Team
LinkOut - more resources
Full Text Sources

