BOP1 contributes to the activation of autophagy in polycystic ovary syndrome via nucleolar stress response
- PMID: 38409361
- PMCID: PMC10896891
- DOI: 10.1007/s00018-023-05091-1
BOP1 contributes to the activation of autophagy in polycystic ovary syndrome via nucleolar stress response
Abstract
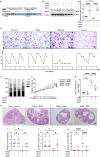
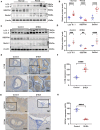
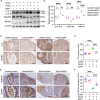
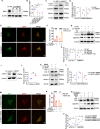
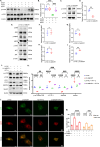
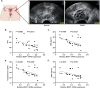
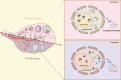
Abnormal autophagy is one of the vital features in polycystic ovary syndrome (PCOS). However, the underlying molecular mechanisms remain unelucidated. In this study, we aimed to investigate whether Block of Proliferation 1 (BOP1) is involved in the onset of autophagy activation of granulosa cells in PCOS. Firstly, we found that BOP1 expression was significantly down-regulated in the ovaries of PCOS mice, which was associated with the development of PCOS. Next, local injection of lentiviral vectors in the ovary for the overexpression of BOP1 significantly alleviated the phenotypes of elevated androgens, disturbed estrous cycle, and abnormal follicular development in PCOS mice. Subsequently, we found that knockdown of BOP1 activated autophagy of granulosa cells in the in vitro experiments, whereas overexpression of BOP1 inhibited autophagy in both in vivo and in vitro models. Mechanistically, BOP1 knockdown triggered the nucleolus stress response, which caused RPL11 to be released from the nucleolus into the nucleoplasm and inhibited the E3 ubiquitination ligase of MDM2, thereby enhancing the stability of p53. Subsequently, P53 inhibited mTOR, thereby activating autophagy in granulosa cells. In addition, the mRNA level of BOP1 was negatively correlated with antral follicle count (AFC), body-mass index (BMI), serum androgen levels, and anti-Mullerian hormone (AMH) in patients with PCOS. In summary, our study demonstrates that BOP1 downregulation inhibits mTOR phosphorylation through activation of the p53-dependent nucleolus stress response, which subsequently contributes to aberrant autophagy in granulosa cells, revealing that BOP1 may be a key target for probing the mechanisms of PCOS.
Keywords: Autophagy; Block of proliferation 1 (BOP1); Nucleolus stress; Polycystic ovary syndrome (PCOS).
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
The phenotypic diversity in per-follicle anti-Müllerian hormone production in polycystic ovary syndrome.Hum Reprod. 2015 Aug;30(8):1927-33. doi: 10.1093/humrep/dev131. Epub 2015 Jun 4. Hum Reprod. 2015. PMID: 26048913
-
Downregulation of CASC15 attenuates the symptoms of polycystic ovary syndrome by affecting granulosa cell proliferation and regulating ovarian follicular development.Mol Cell Endocrinol. 2024 Oct 1;592:112322. doi: 10.1016/j.mce.2024.112322. Epub 2024 Jun 26. Mol Cell Endocrinol. 2024. PMID: 38942281
-
The regulation and signalling of anti-Müllerian hormone in human granulosa cells: relevance to polycystic ovary syndrome.Hum Reprod. 2019 Dec 1;34(12):2467-2479. doi: 10.1093/humrep/dez214. Hum Reprod. 2019. PMID: 31735954
-
Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary.Hum Reprod Update. 2016 Nov;22(6):709-724. doi: 10.1093/humupd/dmw027. Epub 2016 Aug 27. Hum Reprod Update. 2016. PMID: 27566840 Review.
-
Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective.Autophagy. 2021 Oct;17(10):2706-2733. doi: 10.1080/15548627.2021.1938914. Epub 2021 Jun 23. Autophagy. 2021. PMID: 34161185 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- 81971356/National Natural Science Foundation of China
- 81771662/National Natural Science Foundation of China
- 81771618/National Natural Science Foundation of China
- 82271672/National Natural Science Foundation of China
- No. 2016YFC1000600, 2018YFC1002804/National Key Research and Development Program of China
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

