Real-time line-field optical coherence tomography for cellular resolution imaging of biological tissue
- PMID: 38404311
- PMCID: PMC10890841
- DOI: 10.1364/BOE.511187
Real-time line-field optical coherence tomography for cellular resolution imaging of biological tissue
Abstract
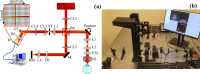
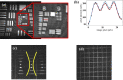
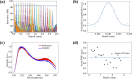
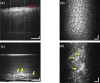
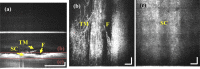
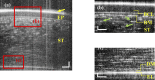
A real-time line-field optical coherence tomography (LF-OCT) system is demonstrated with image acquisition rates of up to 5000 B-frames or 2.5 million A-lines per second for 500 A-lines per B-frame. The system uses a high-speed low-cost camera to achieve continuous data transfer rates required for real-time imaging, allowing the evaluation of future applications in clinical or intraoperative environments. The light source is an 840 nm super-luminescent diode. Leveraging parallel computing with GPU and high speed CoaXPress data transfer interface, we were able to acquire, process, and display OCT data with low latency. The studied system uses anamorphic beam shaping in the detector arm, optimizing the field of view and sensitivity for imaging biological tissue at cellular resolution. The lateral and axial resolution measured in air were 1.7 µm and 6.3 µm, respectively. Experimental results demonstrate real-time inspection of the trabecular meshwork and Schlemm's canal on ex vivo corneoscleral wedges and real-time imaging of endothelial cells of human subjects in vivo.
© 2024 Optica Publishing Group.
Conflict of interest statement
David Huang: Optovue Inc. (F, I, P, R). These potential conflicts of interest have been reviewed and managed by OHSU. Other authors declare no relevant conflicts of interest related to this article.
Figures



Similar articles
-
Schlemm's canal and trabecular meshwork morphology in high myopia.Ophthalmic Physiol Opt. 2018 May;38(3):266-272. doi: 10.1111/opo.12451. Ophthalmic Physiol Opt. 2018. PMID: 29691920
-
Aqueous outflow regulation: Optical coherence tomography implicates pressure-dependent tissue motion.Exp Eye Res. 2017 May;158:171-186. doi: 10.1016/j.exer.2016.06.007. Epub 2016 Jun 11. Exp Eye Res. 2017. PMID: 27302601 Free PMC article. Review.
-
Imaging of human brain tumor tissue by near-infrared laser coherence tomography.Acta Neurochir (Wien). 2009 May;151(5):507-17; discussion 517. doi: 10.1007/s00701-009-0248-y. Epub 2009 Apr 3. Acta Neurochir (Wien). 2009. PMID: 19343270 Free PMC article.
-
Imaging of Ocular Angle Structures with Fourier Domain Optical Coherence Tomography.J Curr Glaucoma Pract. 2013 May-Aug;7(2):85-7. doi: 10.5005/jp-journals-10008-1141. Epub 2013 May 9. J Curr Glaucoma Pract. 2013. PMID: 26997786 Free PMC article.
-
Time-domain full-field optical coherence tomography (TD-FF-OCT) in ophthalmic imaging.Ther Adv Chronic Dis. 2023 May 2;14:20406223231170146. doi: 10.1177/20406223231170146. eCollection 2023. Ther Adv Chronic Dis. 2023. PMID: 37152350 Free PMC article. Review.
Cited by
-
Line-field dynamic optical coherence tomography platform for volumetric assessment of biological tissues.Biomed Opt Express. 2024 Jun 7;15(7):4162-4175. doi: 10.1364/BOE.527797. eCollection 2024 Jul 1. Biomed Opt Express. 2024. PMID: 39022542 Free PMC article.
-
In vivo, contactless, cellular resolution imaging of the human cornea with Powell lens based line field OCT.Sci Rep. 2024 Sep 29;14(1):22553. doi: 10.1038/s41598-024-73402-y. Sci Rep. 2024. PMID: 39343797 Free PMC article.
-
Cellular structural and functional imaging of donor and pathological corneas with label-free dual-mode full-field optical coherence tomography.Biomed Opt Express. 2024 May 21;15(6):3869-3888. doi: 10.1364/BOE.525116. eCollection 2024 Jun 1. Biomed Opt Express. 2024. PMID: 38867788 Free PMC article.
References
-
- Lawman S., Zhang Z., Shen Y.-C., et al. , “Line Field Optical Coherence Tomography,” Photonics 9(12), 946 (2022).10.3390/photonics9120946 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
