Optimized New Shengmai Powder modulation of cAMP/Rap1A signaling pathway attenuates myocardial fibrosis in heart failure
- PMID: 38402401
- PMCID: PMC10894496
- DOI: 10.1186/s13020-024-00902-4
Optimized New Shengmai Powder modulation of cAMP/Rap1A signaling pathway attenuates myocardial fibrosis in heart failure
Abstract
Background: Optimized New Shengmai Powder (ONSMP) is a traditional Chinese medicine formula with significant anti-heart failure and myocardial fibrosis effects, but the specific molecular biological mechanisms are not fully understood.
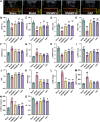
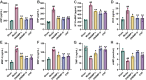
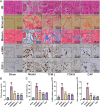
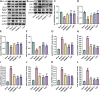
Methods: In this study, we first used network pharmacology to analyze the ONSMP's active ingredients, core signaling pathways, and core targets. Second, calculate the affinity and binding modes of the ONSMP components to the core targets using molecular docking. Finally, the heart failure rat model was established by ligating the left anterior descending branch of the coronary artery and assessing the effect of ONSMP on myocardial fibrosis in heart failure using echocardiography, cardiac organ coefficients, heart failure markers, and pathological sections after 4 weeks of drug intervention. The cAMP level in rat myocardium was determined using Elisa, the α-SMA and FSP-1 positive expression determined by immunohistochemistry, and the protein and mRNA levels of the cAMP/Rap1A signaling pathway were detected by Western Blotting and quantitative real-time PCR, respectively.
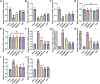
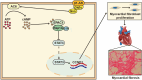
Results: The result shows that the possible mechanism of ONSMP in reducing myocardial fibrosis also includes the use of 12 active ingredients such as baicalin, vitamin D, resveratrol, tanshinone IIA, emodin, 15,16-dihydrotanshinone-i to regulate β1-AR, AC6, EPAC1, Rap1 A, STAT3, and CCND1 on the cAMP/Rap1A signaling pathway, thereby inhibiting the proliferation of cardiac fibroblasts and reduce the excessive secretion of collagen, effectively improve cardiac function and ventricular remodeling in heart failure rats.
Conclusion: This research shows that ONSMP can inhibit myocardial fibrosis and delay heart failure through the cAMP/Rap1A signaling pathway.
Keywords: Heart failure; Myocardial fibrosis; Optimized New Shengmai Powder; Traditional Chinese medicine; cAMP/Rap1A signaling pathway.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Optimized new Shengmai powder ameliorates myocardial fibrosis in rats with heart failure by inhibition of the MAPK signaling pathway.J Ethnopharmacol. 2024 Jan 30;319(Pt 1):117210. doi: 10.1016/j.jep.2023.117210. Epub 2023 Sep 20. J Ethnopharmacol. 2024. PMID: 37739104
-
Regulation of optimized new Shengmai powder on cardiomyocyte apoptosis and ferroptosis in ischemic heart failure rats: The mediating role of phosphatidylinositol-3-kinase/protein kinase B/tumor protein 53 signaling pathway.J Ethnopharmacol. 2024 Aug 10;330:118264. doi: 10.1016/j.jep.2024.118264. Epub 2024 Apr 29. J Ethnopharmacol. 2024. PMID: 38692417
-
Optimized new Shengmai powder inhibits myocardial fibrosis in heart failure by regulating the rat sarcoma/rapidly accelerated fibrosarcoma/mitogen-activated protein kinase kinase/extracellular regulated protein kinases signaling pathway.J Tradit Chin Med. 2024 Jun;44(3):448-457. doi: 10.19852/j.cnki.jtcm.20240402.004. J Tradit Chin Med. 2024. PMID: 38767628 Free PMC article.
-
Effect of optimized new Shengmai powder on exercise tolerance in rats with heart failure by regulating the ubiquitin-proteasome signaling pathway.Front Cardiovasc Med. 2023 May 23;10:1168341. doi: 10.3389/fcvm.2023.1168341. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37288261 Free PMC article.
-
Targeting MAPK-ERK/JNK pathway: A potential intervention mechanism of myocardial fibrosis in heart failure.Biomed Pharmacother. 2024 Apr;173:116413. doi: 10.1016/j.biopha.2024.116413. Epub 2024 Mar 9. Biomed Pharmacother. 2024. PMID: 38461687 Review.
References
-
- Gu D, Huang G, Wu X, Duan X, He J, Whelton PK, et al. Epidemiological survey and prevalence of heart failure in China. Chin J Cardiol. 2003;2003(1):6–9.
-
- Working Group on Heart Failure, National center for cardiovascular quality improvement China heart failure healthcare quality control report. Chin Circ J. 2021;36(3):221–238.
-
- Ma L, Wang Z, Fan J, Hu S. Interpretation of the key points of the china cardiovascular health and disease rport 2022. Chin Gener Pract. 2023;26(32):3975–3994.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

