Safety, Immunogenicity, and Mechanism of a Rotavirus mRNA-LNP Vaccine in Mice
- PMID: 38399987
- PMCID: PMC10892174
- DOI: 10.3390/v16020211
Safety, Immunogenicity, and Mechanism of a Rotavirus mRNA-LNP Vaccine in Mice
Abstract
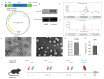
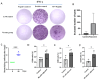
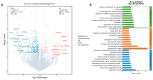
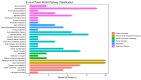
Rotaviruses (RVs) are a major cause of diarrhea in young children worldwide. The currently available and licensed vaccines contain live attenuated RVs. Optimization of live attenuated RV vaccines or developing non-replicating RV (e.g., mRNA) vaccines is crucial for reducing the morbidity and mortality from RV infections. Herein, a nucleoside-modified mRNA vaccine encapsulated in lipid nanoparticles (LNP) and encoding the VP7 protein from the G1 type of RV was developed. The 5' untranslated region of an isolated human RV was utilized for the mRNA vaccine. After undergoing quality inspection, the VP7-mRNA vaccine was injected by subcutaneous or intramuscular routes into mice. Mice received three injections in 21 d intervals. IgG antibodies, neutralizing antibodies, cellular immunity, and gene expression from peripheral blood mononuclear cells were evaluated. Significant differences in levels of IgG antibodies were not observed in groups with adjuvant but were observed in groups without adjuvant. The vaccine without adjuvant induced the highest antibody titers after intramuscular injection. The vaccine elicited a potent antiviral immune response characterized by antiviral clusters of differentiation CD8+ T cells. VP7-mRNA induced interferon-γ secretion to mediate cellular immune responses. Chemokine-mediated signaling pathways and immune response were activated by VP7-mRNA vaccine injection. The mRNA LNP vaccine will require testing for protective efficacy, and it is an option for preventing rotavirus infection.
Keywords: lipid nanoparticles; mRNA vaccine; neutralizing antibody; rotavirus; structural protein VP7.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Inclusion of a universal tetanus toxoid CD4(+) T cell epitope P2 significantly enhanced the immunogenicity of recombinant rotavirus ΔVP8* subunit parenteral vaccines.Vaccine. 2014 Jul 31;32(35):4420-4427. doi: 10.1016/j.vaccine.2014.06.060. Epub 2014 Jun 21. Vaccine. 2014. PMID: 24962749 Free PMC article.
-
Divergence of VP7 genes of G1 rotaviruses isolated from infants vaccinated with reassortant rhesus rotaviruses.Arch Virol. 1996;141(11):2057-76. doi: 10.1007/BF01718215. Arch Virol. 1996. PMID: 8973523 Clinical Trial.
-
Immunogenicity and efficacy in mice of an adenovirus-based bicistronic rotavirus vaccine expressing NSP4 and VP7.Virus Res. 2015 Dec 2;210:298-307. doi: 10.1016/j.virusres.2015.09.010. Epub 2015 Sep 12. Virus Res. 2015. PMID: 26368053
-
Genetic diversity of G1P[8] rotavirus VP7 and VP8* antigens in Finland over a 20-year period: No evidence for selection pressure by universal mass vaccination with RotaTeq® vaccine.Infect Genet Evol. 2013 Oct;19:51-8. doi: 10.1016/j.meegid.2013.06.026. Epub 2013 Jul 4. Infect Genet Evol. 2013. PMID: 23831933 Review.
-
Natural immunity to rotavirus infection in children.Indian J Biochem Biophys. 2008 Aug;45(4):219-28. Indian J Biochem Biophys. 2008. PMID: 18788471 Review.
Cited by
-
Progress and prospects of mRNA-based drugs in pre-clinical and clinical applications.Signal Transduct Target Ther. 2024 Nov 14;9(1):322. doi: 10.1038/s41392-024-02002-z. Signal Transduct Target Ther. 2024. PMID: 39543114 Free PMC article. Review.
References
-
- Tate J.E., Burton A.H., Boschi-Pinto C., Steele A.D., Duque J., Parashar U.D. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: A systematic review and meta-analysis. Lancet Infect. Dis. 2012;12:136–141. doi: 10.1016/S1473-3099(11)70253-5. - DOI - PubMed
-
- Wang H., Naghavi M., Allen C., Barber R.M., Bhutta Z.A., Carter A., Casey D.C., Charlson F.J., Chen A.Z., Coates M.M., et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 202202AA100006/Major Science and Technology Special Project of Yunnan Province (Biomedicine)
- 202201AT070236/Science and Technology Project of Yunnan Province-general program
- 2021-I2M-1-043/CAMS Innovation Fund for Medical Sciences (CIFMS)
- 2019FB020/Science and Technology Project of Yunnan Province-general program
- 202002AA100009/Yunnan Province Innovative Vaccine Technology and Industrial Transformation Platform
LinkOut - more resources
Full Text Sources
Medical
Research Materials

