Recent Technological and Intellectual Property Trends in Antibody-Drug Conjugate Research
- PMID: 38399275
- PMCID: PMC10892729
- DOI: 10.3390/pharmaceutics16020221
Recent Technological and Intellectual Property Trends in Antibody-Drug Conjugate Research
Abstract
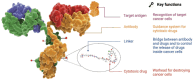
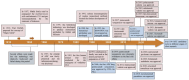
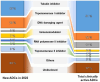
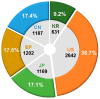
Antibody-drug conjugate (ADC) therapy, an advanced therapeutic technology comprising antibodies, chemical linkers, and cytotoxic payloads, addresses the limitations of traditional chemotherapy. This study explores key elements of ADC therapy, focusing on antibody development, linker design, and cytotoxic payload delivery. The global rise in cancer incidence has driven increased investment in anticancer agents, resulting in significant growth in the ADC therapy market. Over the past two decades, notable progress has been made, with approvals for 14 ADC treatments targeting various cancers by 2022. Diverse ADC therapies for hematologic malignancies and solid tumors have emerged, with numerous candidates currently undergoing clinical trials. Recent years have seen a noteworthy increase in ADC therapy clinical trials, marked by the initiation of numerous new therapies in 2022. Research and development, coupled with patent applications, have intensified, notably from major companies like Pfizer Inc. (New York, NY, USA), AbbVie Pharmaceuticals Inc. (USA), Regeneron Pharmaceuticals Inc. (Tarrytown, NY, USA), and Seagen Inc. (Bothell, WA, USA). While ADC therapy holds great promise in anticancer treatment, challenges persist, including premature payload release and immune-related side effects. Ongoing research and innovation are crucial for advancing ADC therapy. Future developments may include novel conjugation methods, stable linker designs, efficient payload delivery technologies, and integration with nanotechnology, driving the evolution of ADC therapy in anticancer treatment.
Keywords: antibody–drug conjugates (ADCs); innovation; intellectual property (IP) landscape; key players; market; technology trends.
Conflict of interest statement
The manuscript submitted here has not been published or presented elsewhere, either in part or in its entirety, and is not currently under consideration by any other journal. We have thoroughly reviewed and understood the policies of your journal, and we affirm that this manuscript and the study described within it comply with these policies. Additionally, we declare that there are no conflicts of interest associated with this submission.
Figures

Similar articles
-
Antibody drug conjugates beyond cytotoxic payloads.Prog Med Chem. 2023;62:1-59. doi: 10.1016/bs.pmch.2023.10.001. Epub 2023 Nov 14. Prog Med Chem. 2023. PMID: 37981349
-
[Novel Chemical Linkers for Next-generation Antibody-drug Conjugates(ADCs)].Yakugaku Zasshi. 2019;139(2):209-219. doi: 10.1248/yakushi.18-00169-3. Yakugaku Zasshi. 2019. PMID: 30713230 Review. Japanese.
-
Antibody-drug conjugates for breast cancer: a bibliometric study and clinical trial analysis.Discov Oncol. 2024 Aug 2;15(1):329. doi: 10.1007/s12672-024-01192-w. Discov Oncol. 2024. PMID: 39093344 Free PMC article.
-
Advances with antibody-drug conjugates in breast cancer treatment.Eur J Pharm Biopharm. 2021 Dec;169:241-255. doi: 10.1016/j.ejpb.2021.10.016. Epub 2021 Nov 5. Eur J Pharm Biopharm. 2021. PMID: 34748933 Review.
-
The Chemical Design and Synthesis of Linkers Used in Antibody Drug Conjugates.Curr Top Med Chem. 2017;17(32):3393-3424. doi: 10.2174/1568026618666180118155847. Curr Top Med Chem. 2017. PMID: 29357801 Review.
Cited by
-
Antibody-drug conjugates in cancer therapy: mechanisms and clinical studies.MedComm (2020). 2024 Jul 28;5(8):e671. doi: 10.1002/mco2.671. eCollection 2024 Aug. MedComm (2020). 2024. PMID: 39070179 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

