Kcs1 and Vip1: The Key Enzymes behind Inositol Pyrophosphate Signaling in Saccharomyces cerevisiae
- PMID: 38397389
- PMCID: PMC10886477
- DOI: 10.3390/biom14020152
Kcs1 and Vip1: The Key Enzymes behind Inositol Pyrophosphate Signaling in Saccharomyces cerevisiae
Abstract
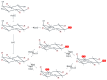
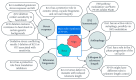
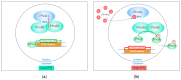
The inositol pyrophosphate pathway, a complex cell signaling network, plays a pivotal role in orchestrating vital cellular processes in the budding yeast, where it regulates cell cycle progression, growth, endocytosis, exocytosis, apoptosis, telomere elongation, ribosome biogenesis, and stress responses. This pathway has gained significant attention in pharmacology and medicine due to its role in generating inositol pyrophosphates, which serve as crucial signaling molecules not only in yeast, but also in higher eukaryotes. As targets for therapeutic development, genetic modifications within this pathway hold promise for disease treatment strategies, offering practical applications in biotechnology. The model organism Saccharomyces cerevisiae, renowned for its genetic tractability, has been instrumental in various studies related to the inositol pyrophosphate pathway. This review is focused on the Kcs1 and Vip1, the two enzymes involved in the biosynthesis of inositol pyrophosphate in S. cerevisiae, highlighting their roles in various cell processes, and providing an up-to-date overview of their relationship with phosphate homeostasis. Moreover, the review underscores the potential applications of these findings in the realms of medicine and biotechnology, highlighting the profound implications of comprehending this intricate signaling network.
Keywords: Kcs1; Saccharomyces cerevisiae; Vip1; inositol hexakisphosphate kinase; inositol pyrophosphate.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Inositol pyrophosphates modulate S phase progression after pheromone-induced arrest in Saccharomyces cerevisiae.J Biol Chem. 2013 Jan 18;288(3):1717-25. doi: 10.1074/jbc.M112.412288. Epub 2012 Nov 24. J Biol Chem. 2013. PMID: 23179856 Free PMC article.
-
Inositol pyrophosphates modulate hydrogen peroxide signalling.Biochem J. 2009 Sep 14;423(1):109-18. doi: 10.1042/BJ20090241. Biochem J. 2009. PMID: 19614566
-
Regulation of inositol metabolism is fine-tuned by inositol pyrophosphates in Saccharomyces cerevisiae.J Biol Chem. 2013 Aug 23;288(34):24898-908. doi: 10.1074/jbc.M113.493353. Epub 2013 Jul 2. J Biol Chem. 2013. PMID: 23824185 Free PMC article.
-
Roles of inositol phosphates and inositol pyrophosphates in development, cell signaling and nuclear processes.Adv Enzyme Regul. 2010;50(1):324-37. doi: 10.1016/j.advenzreg.2009.12.002. Epub 2009 Dec 16. Adv Enzyme Regul. 2010. PMID: 20006638 Free PMC article. Review. No abstract available.
-
The intersection between stress responses and inositol pyrophosphates in Saccharomyces cerevisiae.Curr Genet. 2020 Oct;66(5):901-910. doi: 10.1007/s00294-020-01078-8. Epub 2020 Apr 23. Curr Genet. 2020. PMID: 32322930 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

