Eugenol Suppresses Platelet Activation and Mitigates Pulmonary Thromboembolism in Humans and Murine Models
- PMID: 38396774
- PMCID: PMC10888574
- DOI: 10.3390/ijms25042098
Eugenol Suppresses Platelet Activation and Mitigates Pulmonary Thromboembolism in Humans and Murine Models
Abstract
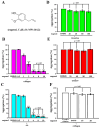
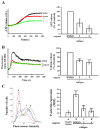
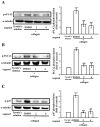
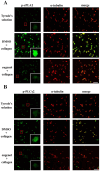
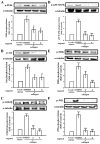
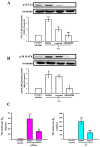
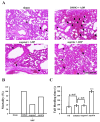
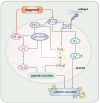
Platelets assume a pivotal role in the pathogenesis of cardiovascular diseases (CVDs), emphasizing their significance in disease progression. Consequently, addressing CVDs necessitates a targeted approach focused on mitigating platelet activation. Eugenol, predominantly derived from clove oil, is recognized for its antibacterial, anticancer, and anti-inflammatory properties, rendering it a valuable medicinal agent. This investigation delves into the intricate mechanisms through which eugenol influences human platelets. At a low concentration of 2 μM, eugenol demonstrates inhibition of collagen and arachidonic acid (AA)-induced platelet aggregation. Notably, thrombin and U46619 remain unaffected by eugenol. Its modulatory effects extend to ATP release, P-selectin expression, and intracellular calcium levels ([Ca2+]i). Eugenol significantly inhibits various signaling cascades, including phospholipase Cγ2 (PLCγ2)/protein kinase C (PKC), phosphoinositide 3-kinase/Akt/glycogen synthase kinase-3β, mitogen-activated protein kinases, and cytosolic phospholipase A2 (cPLA2)/thromboxane A2 (TxA2) formation induced by collagen. Eugenol selectively inhibited cPLA2/TxA2 phosphorylation induced by AA, not affecting p38 MAPK. In ADP-treated mice, eugenol reduced occluded lung vessels by platelet thrombi without extending bleeding time. In conclusion, eugenol exerts a potent inhibitory effect on platelet activation, achieved through the inhibition of the PLCγ2-PKC and cPLA2-TxA2 cascade, consequently suppressing platelet aggregation. These findings underscore the potential therapeutic applications of eugenol in CVDs.
Keywords: MAPK; PI3K/Akt/GSK-3β; PLCγ2–PKC; cPLA2/TxA2; eugenol; human platelets; pulmonary thrombosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Eugenol: A Potential Modulator of Human Platelet Activation and Mouse Mesenteric Vascular Thrombosis via an Innovative cPLA2-NF-κB Signaling Axis.Biomedicines. 2024 Jul 29;12(8):1689. doi: 10.3390/biomedicines12081689. Biomedicines. 2024. PMID: 39200154 Free PMC article.
-
Ginkgetin effectively mitigates collagen and AA-induced platelet activation via PLCγ2 but not cyclic nucleotide-dependent pathway in human.J Cell Mol Med. 2024 Feb;28(4):e18139. doi: 10.1111/jcmm.18139. J Cell Mol Med. 2024. PMID: 38334198 Free PMC article.
-
Glabridin, a Bioactive Flavonoid from Licorice, Effectively Inhibits Platelet Activation in Humans and Mice.Int J Mol Sci. 2022 Sep 27;23(19):11372. doi: 10.3390/ijms231911372. Int J Mol Sci. 2022. PMID: 36232674 Free PMC article.
-
Esculetin, a Coumarin Derivative, Prevents Thrombosis: Inhibitory Signaling on PLCγ2-PKC-AKT Activation in Human Platelets.Int J Mol Sci. 2019 Jun 3;20(11):2731. doi: 10.3390/ijms20112731. Int J Mol Sci. 2019. PMID: 31163690 Free PMC article.
-
Targeting cardiovascular risk factors with eugenol: an anti-inflammatory perspective.Inflammopharmacology. 2024 Feb;32(1):307-317. doi: 10.1007/s10787-023-01392-w. Epub 2023 Dec 12. Inflammopharmacology. 2024. PMID: 38085446 Review.
Cited by
-
Eugenol: A Potential Modulator of Human Platelet Activation and Mouse Mesenteric Vascular Thrombosis via an Innovative cPLA2-NF-κB Signaling Axis.Biomedicines. 2024 Jul 29;12(8):1689. doi: 10.3390/biomedicines12081689. Biomedicines. 2024. PMID: 39200154 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

