Inhibitors of NAD+ Production in Cancer Treatment: State of the Art and Perspectives
- PMID: 38396769
- PMCID: PMC10889166
- DOI: 10.3390/ijms25042092
Inhibitors of NAD+ Production in Cancer Treatment: State of the Art and Perspectives
Abstract
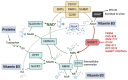
The addiction of tumors to elevated nicotinamide adenine dinucleotide (NAD+) levels is a hallmark of cancer metabolism. Obstructing NAD+ biosynthesis in tumors is a new and promising antineoplastic strategy. Inhibitors developed against nicotinamide phosphoribosyltransferase (NAMPT), the main enzyme in NAD+ production from nicotinamide, elicited robust anticancer activity in preclinical models but not in patients, implying that other NAD+-biosynthetic pathways are also active in tumors and provide sufficient NAD+ amounts despite NAMPT obstruction. Recent studies show that NAD+ biosynthesis through the so-called "Preiss-Handler (PH) pathway", which utilizes nicotinate as a precursor, actively operates in many tumors and accounts for tumor resistance to NAMPT inhibitors. The PH pathway consists of three sequential enzymatic steps that are catalyzed by nicotinate phosphoribosyltransferase (NAPRT), nicotinamide mononucleotide adenylyltransferases (NMNATs), and NAD+ synthetase (NADSYN1). Here, we focus on these enzymes as emerging targets in cancer drug discovery, summarizing their reported inhibitors and describing their current or potential exploitation as anticancer agents. Finally, we also focus on additional NAD+-producing enzymes acting in alternative NAD+-producing routes that could also be relevant in tumors and thus become viable targets for drug discovery.
Keywords: NAD+; NADSYN; NAMPT; NAPRT; NMNAT; Preiss-Handler pathway; cancer metabolism; inhibitors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Advances in NAD-Lowering Agents for Cancer Treatment.Nutrients. 2021 May 14;13(5):1665. doi: 10.3390/nu13051665. Nutrients. 2021. PMID: 34068917 Free PMC article. Review.
-
A Versatile Continuous Fluorometric Enzymatic Assay for Targeting Nicotinate Phosphoribosyltransferase.Molecules. 2023 Jan 18;28(3):961. doi: 10.3390/molecules28030961. Molecules. 2023. PMID: 36770640 Free PMC article.
-
Supplementation of nicotinic acid with NAMPT inhibitors results in loss of in vivo efficacy in NAPRT1-deficient tumor models.Neoplasia. 2013 Dec;15(12):1314-29. doi: 10.1593/neo.131718. Neoplasia. 2013. PMID: 24403854 Free PMC article.
-
Nicotinic Acid Phosphoribosyltransferase Regulates Cancer Cell Metabolism, Susceptibility to NAMPT Inhibitors, and DNA Repair.Cancer Res. 2017 Jul 15;77(14):3857-3869. doi: 10.1158/0008-5472.CAN-16-3079. Epub 2017 May 15. Cancer Res. 2017. PMID: 28507103
-
Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) in cancer: a patent review.Expert Opin Ther Pat. 2024 Jul;34(7):565-582. doi: 10.1080/13543776.2024.2367006. Epub 2024 Jun 13. Expert Opin Ther Pat. 2024. PMID: 38861278 Review.
Cited by
-
Synthesis, Biological, and Computational Evaluations of Conformationally Restricted NAD-Mimics as Discriminant Inhibitors of Human NMN-Adenylyltransferase Isozymes.Pharmaceuticals (Basel). 2024 Jun 6;17(6):739. doi: 10.3390/ph17060739. Pharmaceuticals (Basel). 2024. PMID: 38931406 Free PMC article.
-
Biological Functions and Therapeutic Potential of NAD+ Metabolism in Gynecological Cancers.Cancers (Basel). 2024 Sep 5;16(17):3085. doi: 10.3390/cancers16173085. Cancers (Basel). 2024. PMID: 39272943 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

