Malat1 regulates PMN-MDSC expansion and immunosuppression through p-STAT3 ubiquitination in sepsis
- PMID: 38385073
- PMCID: PMC10878150
- DOI: 10.7150/ijbs.92267
Malat1 regulates PMN-MDSC expansion and immunosuppression through p-STAT3 ubiquitination in sepsis
Abstract
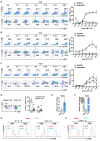
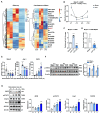
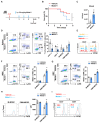
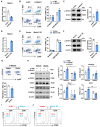
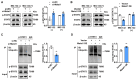
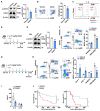
Myeloid-derived suppressor cells (MDSCs) expand during sepsis and contribute to the development of persistent inflammation-immunosuppression-catabolism syndrome. However, the underlying mechanism remains unclear. Exploring the mechanisms of MDSCs generation may provide therapeutic targets for improving immune status in sepsis. Here, a sepsis mouse model is established by cecal ligation and perforation. Bone marrow cells at different sepsis time points are harvested to detect the proportion of MDSCs and search for differentially expressed genes by RNA-sequence. In lethal models of sepsis, polymorphonuclear-MDSCs (PMN-MDSCs) decrease in early but increase and become activated in late sepsis, which is contrary to the expression of metastasis-associated lung adenocarcinoma transcript 1 (Malat1). In vivo, Malat1 inhibitor significantly increases the mortality in mice with late sepsis. And in vitro, Malat1 down-regulation increases the proportion of PMN-MDSCs and enhanced its immunosuppressive ability. Mechanistically, Malat1 limits the differentiation of PMN-MDSCs by accelerating the degradation of phosphorylated STAT3. Furthermore, Stattic, an inhibitor of STAT3 phosphorylation, improves the survival of septic mice by inhibiting PMN-MDSCs. Overall, the study identifies a novel insight into the mechanism of sepsis-induced MDSCs and provides more evidence for targeting MDSCs in the treatment of sepsis.
Keywords: Experimental sepsis; Malat1; Polymorphonuclear myeloid-derived suppressor cell; STAT3 pathway; Ubiquitination.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Early Activation of Myeloid-Derived Suppressor Cells Participate in Sepsis-Induced Immune Suppression via PD-L1/PD-1 Axis.Front Immunol. 2020 Jul 3;11:1299. doi: 10.3389/fimmu.2020.01299. eCollection 2020. Front Immunol. 2020. PMID: 32719675 Free PMC article.
-
LDK378 inhibits the recruitment of myeloid-derived suppressor cells to spleen via the p38-GRK2-CCR2 pathway in mice with sepsis.Immunol Cell Biol. 2019 Nov;97(10):902-915. doi: 10.1111/imcb.12289. Epub 2019 Oct 6. Immunol Cell Biol. 2019. PMID: 31472096
-
PMN-MDSCs Enhance CTC Metastatic Properties through Reciprocal Interactions via ROS/Notch/Nodal Signaling.Int J Mol Sci. 2019 Apr 18;20(8):1916. doi: 10.3390/ijms20081916. Int J Mol Sci. 2019. PMID: 31003475 Free PMC article.
-
Myeloid-Derived Suppressor Cells in Sepsis.Front Immunol. 2019 Feb 27;10:327. doi: 10.3389/fimmu.2019.00327. eCollection 2019. Front Immunol. 2019. PMID: 30873175 Free PMC article. Review.
-
MDSCs in sepsis-induced immunosuppression and its potential therapeutic targets.Cytokine Growth Factor Rev. 2023 Feb;69:90-103. doi: 10.1016/j.cytogfr.2022.07.007. Epub 2022 Jul 21. Cytokine Growth Factor Rev. 2023. PMID: 35927154 Review.
Cited by
-
Long noncoding RNA MALAT-1: A versatile regulator in cancer progression, metastasis, immunity, and therapeutic resistance.Noncoding RNA Res. 2024 Feb 1;9(2):388-406. doi: 10.1016/j.ncrna.2024.01.015. eCollection 2024 Jun. Noncoding RNA Res. 2024. PMID: 38511067 Free PMC article. Review.
References
-
- Xie J, Wang H, Kang Y. et al. The Epidemiology of Sepsis in Chinese ICUs: A National Cross-Sectional Survey. Crit Care Med. 2020;48(3):E209–E218. - PubMed
-
- Van Der Poll T, Van De Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17(7):407–420. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

