Sirtuin 4 (Sirt4) downregulation contributes to chondrocyte senescence and osteoarthritis via mediating mitochondrial dysfunction
- PMID: 38385071
- PMCID: PMC10878156
- DOI: 10.7150/ijbs.85585
Sirtuin 4 (Sirt4) downregulation contributes to chondrocyte senescence and osteoarthritis via mediating mitochondrial dysfunction
Abstract
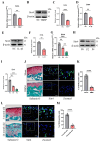
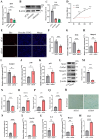
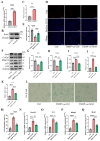
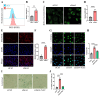
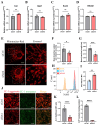
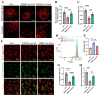
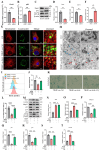
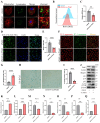
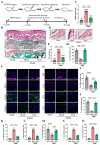
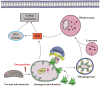
Chondrocyte senescence has recently been proposed as a key pathogenic mechanism in the etiology of osteoarthritis (OA). Nevertheless, the precise molecular mechanisms underlying chondrocyte senescence remain poorly understood. To address this knowledge gap, we conducted an investigation into the involvement of Sirtuin 4 (Sirt4) in chondrocyte senescence. Our experimental findings revealed a downregulation of Sirt4 expression in TBHP-induced senescent chondrocytes in vitro, as well as in mouse OA cartilage. Additionally, we observed that the knockdown of Sirt4 in chondrocytes promoted cellular senescence and cartilage degradation, while the overexpression of Sirt4 protected the cells against TBHP-mediated senescence of chondrocytes and cartilage degradation. Moreover, our findings revealed elevated levels of reactive oxygen species (ROS), abnormal mitochondrial morphology, compromised mitochondrial membrane potential, and reduced ATP production in Sirt4 knockdown chondrocytes, indicative of mitochondrial dysfunction. Conversely, Sirt4 overexpression successfully mitigated TBHP-induced mitochondrial dysfunction. Further analysis revealed that Sirt4 downregulation impaired the cellular capacity to eliminate damaged mitochondria by inhibiting Pink1 in chondrocytes, thereby enhancing the accumulation of ROS and facilitating chondrocyte senescence. Notably, the overexpression of Pink1 counteracted the effects of Sirt4 knockdown on mitochondrial dysfunction. Importantly, our study demonstrated the promise of gene therapy employing a lentiviral vector encoding mouse Sirt4, as it successfully preserved the integrity of articular cartilage in mouse models of OA. In conclusion, our findings provide compelling evidence that the overexpression of Sirt4 enhances mitophagy, restores mitochondrial function, and protects against chondrocyte senescence, thereby offering a novel therapeutic target and potential strategy for the treatment of OA.
Keywords: Mitochondria; Osteoarthritis; Reactive oxygen species; Senescence; Sirt4.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
PDZK1 protects against mechanical overload-induced chondrocyte senescence and osteoarthritis by targeting mitochondrial function.Bone Res. 2024 Jul 17;12(1):41. doi: 10.1038/s41413-024-00344-6. Bone Res. 2024. PMID: 39019845 Free PMC article.
-
Protocatechuic aldehyde attenuates chondrocyte senescence via the regulation of PTEN-induced kinase 1/Parkin-mediated mitochondrial autophagy.Int J Immunopathol Pharmacol. 2024 Jan-Dec;38:3946320241271724. doi: 10.1177/03946320241271724. Int J Immunopathol Pharmacol. 2024. PMID: 39116410 Free PMC article.
-
Inhibition of Cpt1a alleviates oxidative stress-induced chondrocyte senescence via regulating mitochondrial dysfunction and activating mitophagy.Mech Ageing Dev. 2022 Jul;205:111688. doi: 10.1016/j.mad.2022.111688. Epub 2022 Jun 18. Mech Ageing Dev. 2022. PMID: 35728631
-
The role of the sirtuin family in cartilage and osteoarthritis: molecular mechanisms and therapeutic targets.Arthritis Res Ther. 2022 Dec 31;24(1):286. doi: 10.1186/s13075-022-02983-8. Arthritis Res Ther. 2022. PMID: 36585687 Free PMC article. Review.
-
Reactive oxygen species, aging and articular cartilage homeostasis.Free Radic Biol Med. 2019 Feb 20;132:73-82. doi: 10.1016/j.freeradbiomed.2018.08.038. Epub 2018 Aug 31. Free Radic Biol Med. 2019. PMID: 30176344 Free PMC article. Review.
Cited by
-
Cellular Senescence: The Driving Force of Musculoskeletal Diseases.Biomedicines. 2024 Aug 26;12(9):1948. doi: 10.3390/biomedicines12091948. Biomedicines. 2024. PMID: 39335461 Free PMC article. Review.
-
Characterizing mitochondrial features in osteoarthritis through integrative multi-omics and machine learning analysis.Front Immunol. 2024 Jul 4;15:1414301. doi: 10.3389/fimmu.2024.1414301. eCollection 2024. Front Immunol. 2024. PMID: 39026663 Free PMC article.
-
10-hydroxy-2-decenoic acid prevents osteoarthritis by targeting aspartyl β hydroxylase and inhibiting chondrocyte senescence in male mice preclinically.Nat Commun. 2024 Sep 4;15(1):7712. doi: 10.1038/s41467-024-51746-3. Nat Commun. 2024. PMID: 39231947 Free PMC article.
References
-
- Sharma L. Osteoarthritis of the Knee. N Engl J Med. 2021;384(1):51–59. - PubMed
-
- Zhao X, Shah D, Gandhi K. et al. Clinical, humanistic, and economic burden of osteoarthritis among noninstitutionalized adults in the United States. Osteoarthritis Cartilage. 2019;27(11):1618–1626. - PubMed
-
- Yue L, Berman J. What Is Osteoarthritis? Jama. 2022;327(13):1300. - PubMed
-
- Liu Q, Wang S, Lin J, Zhang Y. The burden for knee osteoarthritis among Chinese elderly: estimates from a nationally representative study. Osteoarthritis Cartilage. 2018;26(12):1636–1642. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

