Innate and adaptive immune-directed tumour microenvironment in pancreatic ductal adenocarcinoma
- PMID: 38384463
- PMCID: PMC10879611
- DOI: 10.3389/fimmu.2024.1323198
Innate and adaptive immune-directed tumour microenvironment in pancreatic ductal adenocarcinoma
Abstract
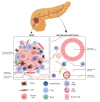
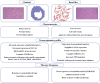
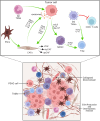
One of the most deadly and aggressive cancers in the world, pancreatic ductal adenocarcinoma (PDAC), typically manifests at an advanced stage. PDAC is becoming more common, and by the year 2030, it is expected to overtake lung cancer as the second greatest cause of cancer-related death. The poor prognosis can be attributed to a number of factors, including difficulties in early identification, a poor probability of curative radical resection, limited response to chemotherapy and radiotherapy, and its immunotherapy resistance. Furthermore, an extensive desmoplastic stroma that surrounds PDAC forms a mechanical barrier that prevents vascularization and promotes poor immune cell penetration. Phenotypic heterogeneity, drug resistance, and immunosuppressive tumor microenvironment are the main causes of PDAC aggressiveness. There is a complex and dynamic interaction between tumor cells in PDAC with stromal cells within the tumour immune microenvironment. The immune suppressive microenvironment that promotes PDAC aggressiveness is contributed by a range of cellular and humoral factors, which itself are modulated by the cancer. In this review, we describe the role of innate and adaptive immune cells, complex tumor microenvironment in PDAC, humoral factors, innate immune-mediated therapeutic advances, and recent clinical trials in PDAC.
Keywords: EMT; PDAC; TME; TNF-α; immune suppression; immune surveillance; macrophages.
Copyright © 2024 Joseph, Al Aiyan, Al-Ramadi, Singh and Kishore.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
Current advances and outlooks in immunotherapy for pancreatic ductal adenocarcinoma.Mol Cancer. 2020 Feb 15;19(1):32. doi: 10.1186/s12943-020-01151-3. Mol Cancer. 2020. PMID: 32061257 Free PMC article. Review.
-
The reciprocal regulation between host tissue and immune cells in pancreatic ductal adenocarcinoma: new insights and therapeutic implications.Mol Cancer. 2019 Dec 13;18(1):184. doi: 10.1186/s12943-019-1117-9. Mol Cancer. 2019. PMID: 31831007 Free PMC article. Review.
-
The Role of Tumor Microenvironment in Pancreatic Cancer Immunotherapy: Current Status and Future Perspectives.Int J Mol Sci. 2024 Sep 3;25(17):9555. doi: 10.3390/ijms25179555. Int J Mol Sci. 2024. PMID: 39273502 Free PMC article. Review.
-
Defining the spatial distribution of extracellular adenosine revealed a myeloid-dependent immunosuppressive microenvironment in pancreatic ductal adenocarcinoma.J Immunother Cancer. 2023 Aug;11(8):e006457. doi: 10.1136/jitc-2022-006457. J Immunother Cancer. 2023. PMID: 37553182 Free PMC article.
-
Pancreatic Tumor Microenvironment.Adv Exp Med Biol. 2020;1296:243-257. doi: 10.1007/978-3-030-59038-3_15. Adv Exp Med Biol. 2020. PMID: 34185297
Cited by
-
Biological Barriers for Drug Delivery and Development of Innovative Therapeutic Approaches in HIV, Pancreatic Cancer, and Hemophilia A/B.Pharmaceutics. 2024 Sep 13;16(9):1207. doi: 10.3390/pharmaceutics16091207. Pharmaceutics. 2024. PMID: 39339243 Free PMC article. Review.
-
CXCL6-CXCR2 axis-mediated PD-L2+ mast cell accumulation shapes the immunosuppressive microenvironment in osteosarcoma.Heliyon. 2024 Jul 8;10(14):e34290. doi: 10.1016/j.heliyon.2024.e34290. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39082021 Free PMC article.
-
A mathematical model for pancreatic cancer during intraepithelial neoplasia.R Soc Open Sci. 2024 Oct 30;11(10):240702. doi: 10.1098/rsos.240702. eCollection 2024 Oct. R Soc Open Sci. 2024. PMID: 39493299 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

