Development and validation of cryopreserved or freeze-dried decellularized human dermis for transplantation
- PMID: 38381276
- PMCID: PMC11143058
- DOI: 10.1007/s10561-024-10131-6
Development and validation of cryopreserved or freeze-dried decellularized human dermis for transplantation
Abstract
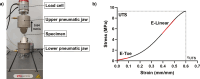
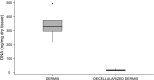
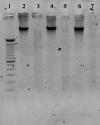
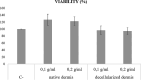
For decades, dermal tissue grafts have been used in various regenerative, reconstructive, and augmentative procedures across the body. To eliminate antigenicity and immunogenic response while still preserving the individual components and collective structural integrity of the extracellular matrix (ECM), dermis can be decellularized. Acellular dermal matrix (ADM) products like such are produced to accurately serve diverse clinical purposes. The aim of the present study is to evaluate the efficacy of a novel decellularization protocol of the human dermis, which eliminates residual human genetic material without compromising the biomechanical integrity and collagenous content of the tissue. Moreover, a freeze-drying protocol was validated. The results showed that though our decellularization protocol, human dermis can be decellularized obtaining a biocompatible matrix. The procedure is completely realized in GMP aseptic condition, avoiding tissue terminal sterilization.
Keywords: Acellular dermal matrix; Allograft; Decellularization; Decellularized dermis; Tissue bank.
© 2024. The Author(s).
Conflict of interest statement
The authors have no competing interests to declare that are relevant to the content of this article.
Figures


Similar articles
-
Fast protocol for the processing of split-thickness skin into decellularized human dermal matrix.Tissue Cell. 2021 Oct;72:101572. doi: 10.1016/j.tice.2021.101572. Epub 2021 Jun 4. Tissue Cell. 2021. PMID: 34119882
-
Decellularization and preservation of human skin: A platform for tissue engineering and reconstructive surgery.Methods. 2020 Jan 15;171:62-67. doi: 10.1016/j.ymeth.2019.07.005. Epub 2019 Jul 11. Methods. 2020. PMID: 31302179
-
Histological evaluation of decellularization of freeze dried and chemically treated indigenously prepared bovine pericardium membrane.Cell Tissue Bank. 2024 Sep;25(3):773-784. doi: 10.1007/s10561-024-10139-y. Epub 2024 May 23. Cell Tissue Bank. 2024. PMID: 38780817
-
Decellularization of human dermis using non-denaturing anionic detergent and endonuclease: a review.Cell Tissue Bank. 2015 Jun;16(2):249-59. doi: 10.1007/s10561-014-9467-4. Epub 2014 Aug 28. Cell Tissue Bank. 2015. PMID: 25163609 Free PMC article. Review.
-
Regulation of decellularized matrix mediated immune response.Biomater Sci. 2020 Mar 3;8(5):1194-1215. doi: 10.1039/c9bm01780a. Biomater Sci. 2020. PMID: 31930231 Review.
References
-
- Bondioli E, Purpura V, Orlandi C, Carboni A, Minghetti P, Cenacchi G, De Luca G, Capirossi D, Nigrisoli E, Melandri D. The use of an acellular matrix derived from human dermis for the treatment of full-thickness skin wounds. Cell Tissue Bank. 2019;20(2):183–192. doi: 10.1007/s10561-019-09755-w. - DOI - PubMed
-
- Bottino MC, Jose MV, Thomas V, Dean DR, Janowski GM. Freeze-dried acellular dermal matrix graft: effects of rehydration on physical, chemical, and mechanical properties. Dental Materials: Official Publication of the Academy of Dental Material. 2009;25(9):1109–1115. doi: 10.1016/j.dental.2009.03.007. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

