Mechanisms of endothelial activation, hypercoagulation and thrombosis in COVID-19: a link with diabetes mellitus
- PMID: 38378550
- PMCID: PMC10880237
- DOI: 10.1186/s12933-023-02097-8
Mechanisms of endothelial activation, hypercoagulation and thrombosis in COVID-19: a link with diabetes mellitus
Abstract
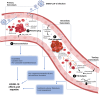
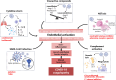
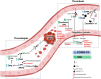
Early since the onset of the COVID-19 pandemic, the medical and scientific community were aware of extra respiratory actions of SARS-CoV-2 infection. Endothelitis, hypercoagulation, and hypofibrinolysis were identified in COVID-19 patients as subsequent responses of endothelial dysfunction. Activation of the endothelial barrier may increase the severity of the disease and contribute to long-COVID syndrome and post-COVID sequelae. Besides, it may cause alterations in primary, secondary, and tertiary hemostasis. Importantly, these responses have been highly decisive in the evolution of infected patients also diagnosed with diabetes mellitus (DM), who showed previous endothelial dysfunction. In this review, we provide an overview of the potential triggers of endothelial activation related to COVID-19 and COVID-19 under diabetic milieu. Several mechanisms are induced by both the viral particle itself and by the subsequent immune-defensive response (i.e., NF-κB/NLRP3 inflammasome pathway, vasoactive peptides, cytokine storm, NETosis, activation of the complement system). Alterations in coagulation mediators such as factor VIII, fibrin, tissue factor, the von Willebrand factor: ADAMST-13 ratio, and the kallikrein-kinin or plasminogen-plasmin systems have been reported. Moreover, an imbalance of thrombotic and thrombolytic (tPA, PAI-I, fibrinogen) factors favors hypercoagulation and hypofibrinolysis. In the context of DM, these mechanisms can be exacerbated leading to higher loss of hemostasis. However, a series of therapeutic strategies targeting the activated endothelium such as specific antibodies or inhibitors against thrombin, key cytokines, factor X, complement system, the kallikrein-kinin system or NETosis, might represent new opportunities to address this hypercoagulable state present in COVID-19 and DM. Antidiabetics may also ameliorate endothelial dysfunction, inflammation, and platelet aggregation. By improving the microvascular pathology in COVID-19 and post-COVID subjects, the associated comorbidities and the risk of mortality could be reduced.
Keywords: COVID-19; Coagulation; Diabetes mellitus; Endothelial cells; SARS-CoV-2; Thrombosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Impaired coagulation, liver dysfunction and COVID-19: Discovering an intriguing relationship.World J Gastroenterol. 2022 Mar 21;28(11):1102-1112. doi: 10.3748/wjg.v28.i11.1102. World J Gastroenterol. 2022. PMID: 35431501 Free PMC article. Review.
-
COVID-19 coagulopathy - what should we treat?Exp Physiol. 2022 Jul;107(7):749-758. doi: 10.1113/EP089404. Epub 2022 Jun 22. Exp Physiol. 2022. PMID: 35733235 Free PMC article. Review.
-
Evaluation of COVID-19 coagulopathy; laboratory characterization using thrombin generation and nonconventional haemostasis assays.Int J Lab Hematol. 2021 Feb;43(1):123-130. doi: 10.1111/ijlh.13329. Epub 2020 Sep 5. Int J Lab Hematol. 2021. PMID: 32892505
-
Covid-19: The Rollercoaster of Fibrin(Ogen), D-Dimer, Von Willebrand Factor, P-Selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes.Int J Mol Sci. 2020 Jul 21;21(14):5168. doi: 10.3390/ijms21145168. Int J Mol Sci. 2020. PMID: 32708334 Free PMC article. Review.
-
Thromboplasminflammation in COVID-19 Coagulopathy: Three Viewpoints for Diagnostic and Therapeutic Strategies.Front Immunol. 2021 Jun 11;12:649122. doi: 10.3389/fimmu.2021.649122. eCollection 2021. Front Immunol. 2021. PMID: 34177896 Free PMC article. Review.
Cited by
-
Type 2 Diabetes Mellitus Aggravates Complement Dysregulation and Affects Cortisol Response in Patients with Post-COVID-19.Diabetes Metab Syndr Obes. 2024 Oct 19;17:3849-3861. doi: 10.2147/DMSO.S480457. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 39449862 Free PMC article.
-
Superficial Vein Thrombosis in an Asymptomatic Case of Cholangiocarcinoma with Recent History of COVID-19.Life (Basel). 2024 Aug 30;14(9):1095. doi: 10.3390/life14091095. Life (Basel). 2024. PMID: 39337879 Free PMC article.
-
Good metabolic control is associated with decreased circulating factor VIIa- antithrombin complexes in type 2 diabetes: a cross-sectional study.Cardiovasc Diabetol. 2024 Nov 5;23(1):398. doi: 10.1186/s12933-024-02480-z. Cardiovasc Diabetol. 2024. PMID: 39501309 Free PMC article.
-
Correlating COVID-19 severity with biomarker profiles and patient prognosis.Sci Rep. 2024 Sep 27;14(1):22353. doi: 10.1038/s41598-024-71951-w. Sci Rep. 2024. PMID: 39333538 Free PMC article.
-
COVID-19: a multi-organ perspective.Front Cell Infect Microbiol. 2024 Oct 18;14:1425547. doi: 10.3389/fcimb.2024.1425547. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39492990 Free PMC article. Review.
References
-
- Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

