Designer high-density lipoprotein particles enhance endothelial barrier function and suppress inflammation
- PMID: 38377179
- PMCID: PMC10954247
- DOI: 10.1126/scisignal.adg9256
Designer high-density lipoprotein particles enhance endothelial barrier function and suppress inflammation
Abstract
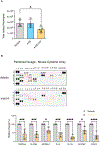
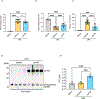
High-density lipoprotein (HDL) nanoparticles promote endothelial cell (EC) function and suppress inflammation, but their utility in treating EC dysfunction has not been fully explored. Here, we describe a fusion protein named ApoA1-ApoM (A1M) consisting of apolipoprotein A1 (ApoA1), the principal structural protein of HDL that forms lipid nanoparticles, and ApoM, a chaperone for the bioactive lipid sphingosine 1-phosphate (S1P). A1M forms HDL-like particles, binds to S1P, and is signaling competent. Molecular dynamics simulations showed that the S1P-bound ApoM moiety in A1M efficiently activated EC surface receptors. Treatment of human umbilical vein ECs with A1M-S1P stimulated barrier function either alone or cooperatively with other barrier-enhancing molecules, including the stable prostacyclin analog iloprost, and suppressed cytokine-induced inflammation. A1M-S1P injection into mice during sterile inflammation suppressed neutrophil influx and inflammatory mediator secretion. Moreover, systemic A1M administration led to a sustained increase in circulating HDL-bound S1P and suppressed inflammation in a murine model of LPS-induced endotoxemia. We propose that A1M administration may enhance vascular endothelial barrier function, suppress cytokine storm, and promote resilience of the vascular endothelium.
Conflict of interest statement
Figures






Similar articles
-
High-Density Lipoprotein-Associated Apolipoprotein M Limits Endothelial Inflammation by Delivering Sphingosine-1-Phosphate to the Sphingosine-1-Phosphate Receptor 1.Arterioscler Thromb Vasc Biol. 2017 Jan;37(1):118-129. doi: 10.1161/ATVBAHA.116.308435. Epub 2016 Nov 22. Arterioscler Thromb Vasc Biol. 2017. PMID: 27879252
-
HDL-associated ApoM is anti-apoptotic by delivering sphingosine 1-phosphate to S1P1 & S1P3 receptors on vascular endothelium.Lipids Health Dis. 2017 Feb 8;16(1):36. doi: 10.1186/s12944-017-0429-2. Lipids Health Dis. 2017. PMID: 28179022 Free PMC article.
-
HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation.Sci Signal. 2015 Aug 11;8(389):ra79. doi: 10.1126/scisignal.aaa2581. Sci Signal. 2015. PMID: 26268607 Free PMC article.
-
Apolipoprotein M and its impact on endothelial dysfunction and inflammation in the cardiovascular system.Atherosclerosis. 2021 Oct;334:76-84. doi: 10.1016/j.atherosclerosis.2021.08.039. Epub 2021 Aug 27. Atherosclerosis. 2021. PMID: 34482091 Review.
-
Sphingosine-1-phosphate: metabolism, transport, atheroprotection and effect of statin treatment.Curr Opin Lipidol. 2022 Jun 1;33(3):199-207. doi: 10.1097/MOL.0000000000000825. Epub 2022 Mar 9. Curr Opin Lipidol. 2022. PMID: 35695616 Review.
Cited by
-
Rapid determination of sphingosine 1-phosphate association with carrier molecules by flow-induced dispersion analysis to predict sepsis outcome.iScience. 2024 Oct 15;27(11):111168. doi: 10.1016/j.isci.2024.111168. eCollection 2024 Nov 15. iScience. 2024. PMID: 39524325 Free PMC article.
References
-
- Pober JS, Sessa WC, Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 7, 803–815 (2007). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

