Listeria adhesion protein orchestrates caveolae-mediated apical junctional remodeling of epithelial barrier for Listeria monocytogenes translocation
- PMID: 38376160
- PMCID: PMC10936185
- DOI: 10.1128/mbio.02821-23
Listeria adhesion protein orchestrates caveolae-mediated apical junctional remodeling of epithelial barrier for Listeria monocytogenes translocation
Abstract
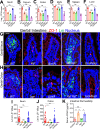
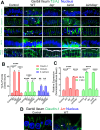
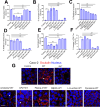
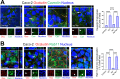
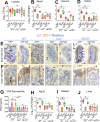
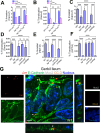
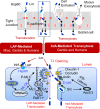
The cellular junctional architecture remodeling by Listeria adhesion protein-heat shock protein 60 (LAP-Hsp60) interaction for Listeria monocytogenes (Lm) passage through the epithelial barrier is incompletely understood. Here, using the gerbil model, permissive to internalin (Inl) A/B-mediated pathways like in humans, we demonstrate that Lm crosses the intestinal villi at 48 h post-infection. In contrast, the single isogenic (lap- or ΔinlA) or double (lap-ΔinlA) mutant strains show significant defects. LAP promotes Lm translocation via endocytosis of cell-cell junctional complex in enterocytes that do not display luminal E-cadherin. In comparison, InlA facilitates Lm translocation at cells displaying apical E-cadherin during cell extrusion and mucus expulsion from goblet cells. LAP hijacks caveolar endocytosis to traffic integral junctional proteins to the early and recycling endosomes. Pharmacological inhibition in a cell line and genetic knockout of caveolin-1 in mice prevents LAP-induced intestinal permeability, junctional endocytosis, and Lm translocation. Furthermore, LAP-Hsp60-dependent tight junction remodeling is also necessary for InlA access to E-cadherin for Lm intestinal barrier crossing in InlA-permissive hosts.
Importance: Listeria monocytogenes (Lm) is a foodborne pathogen with high mortality (20%-30%) and hospitalization rates (94%), particularly affecting vulnerable groups such as pregnant women, fetuses, newborns, seniors, and immunocompromised individuals. Invasive listeriosis involves Lm's internalin (InlA) protein binding to E-cadherin to breach the intestinal barrier. However, non-functional InlA variants have been identified in Lm isolates, suggesting InlA-independent pathways for translocation. Our study reveals that Listeria adhesion protein (LAP) and InlA cooperatively assist Lm entry into the gut lamina propria in a gerbil model, mimicking human listeriosis in early infection stages. LAP triggers caveolin-1-mediated endocytosis of critical junctional proteins, transporting them to early and recycling endosomes, facilitating Lm passage through enterocytes. Furthermore, LAP-Hsp60-mediated junctional protein endocytosis precedes InlA's interaction with basolateral E-cadherin, emphasizing LAP and InlA's cooperation in enhancing Lm intestinal translocation. This understanding is vital in combating the severe consequences of Lm infection, including sepsis, meningitis, encephalitis, and brain abscess.
Keywords: E-cadherin; Listeria adhesion protein (LAP); Listeria monocytogenes (Lm); caveolin; dynamin; gerbil; heat shock protein 60 (Hsp60); internalin A (InlA); intestinal barrier; tight junction (TJ); translocation.
Conflict of interest statement
A patent on LAP use as a tight junction modulator has been issued.
Figures


Similar articles
-
Listeria monocytogenes internalin B activates junctional endocytosis to accelerate intestinal invasion.PLoS Pathog. 2010 May 13;6(5):e1000900. doi: 10.1371/journal.ppat.1000900. PLoS Pathog. 2010. PMID: 20485518 Free PMC article.
-
Listeria Adhesion Protein Induces Intestinal Epithelial Barrier Dysfunction for Bacterial Translocation.Cell Host Microbe. 2018 Apr 11;23(4):470-484.e7. doi: 10.1016/j.chom.2018.03.004. Epub 2018 Apr 5. Cell Host Microbe. 2018. PMID: 29606495 Free PMC article.
-
Murinization of internalin extends its receptor repertoire, altering Listeria monocytogenes cell tropism and host responses.PLoS Pathog. 2013;9(5):e1003381. doi: 10.1371/journal.ppat.1003381. Epub 2013 May 30. PLoS Pathog. 2013. PMID: 23737746 Free PMC article.
-
Crossing the Intestinal Barrier via Listeria Adhesion Protein and Internalin A.Trends Microbiol. 2019 May;27(5):408-425. doi: 10.1016/j.tim.2018.12.007. Epub 2019 Jan 17. Trends Microbiol. 2019. PMID: 30661918 Review.
-
Understanding how Listeria monocytogenes targets and crosses host barriers.Clin Microbiol Infect. 2005 Jun;11(6):430-6. doi: 10.1111/j.1469-0691.2005.01146.x. Clin Microbiol Infect. 2005. PMID: 15882192 Review.
Cited by
-
Current methodologies available to evaluate the virulence potential among Listeria monocytogenes clonal complexes.Front Microbiol. 2024 Oct 10;15:1425437. doi: 10.3389/fmicb.2024.1425437. eCollection 2024. Front Microbiol. 2024. PMID: 39493856 Free PMC article. Review.
-
Listeria in Pregnancy-The Forgotten Culprit.Microorganisms. 2024 Oct 21;12(10):2102. doi: 10.3390/microorganisms12102102. Microorganisms. 2024. PMID: 39458411 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
