Gingival-derived mesenchymal stem cell therapy regenerated the radiated salivary glands: functional and histological evidence in murine model
- PMID: 38365799
- PMCID: PMC10874004
- DOI: 10.1186/s13287-024-03659-7
Gingival-derived mesenchymal stem cell therapy regenerated the radiated salivary glands: functional and histological evidence in murine model
Abstract
Background: Radiotherapy in head and neck cancer management causes degeneration of the salivary glands (SG). This study was designed to determine the potential of gingival mesenchymal stem cells (GMSCs) as a cell-based therapy to regenerate irradiated parotid SG tissues and restore their function using a murine model.
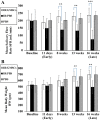
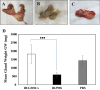
Methods: Cultured isolated cells from gingival tissues of 4 healthy guinea pigs at passage 3 were characterized as GMSCSs using flow cytometry for surface markers and multilineage differentiation capacity. Twenty-one Guinea pigs were equally divided into three groups: Group I/Test, received single local irradiation of 15 Gy to the head and neck field followed by intravenous injection of labeled GMSCs, Group II/Positive control, which received the same irradiation dose followed by injection of phosphate buffer solution (PBS), and Group III/Negative control, received (PBS) injection only. Body weight and salivary flow rate (SFR) were measured at baseline, 11 days, 8-, 13- and 16-weeks post-irradiation. At 16 weeks, parotid glands were harvested for assessment of gland weight and histological and immunohistochemical analysis.
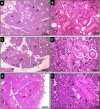
Results: The injected GMSCs homed to degenerated glands, with subsequent restoration of the normal gland histological acinar and tubular structure associated with a significant increase in cell proliferation and reduction in apoptotic activity. Subsequently, a significant increase in body weight and SFR, as well as an increase in gland weight at 16 weeks in comparison with the irradiated non-treated group were observed.
Conclusion: The study provided a new potential therapeutic strategy for the treatment of xerostomia by re-engineering radiated SG using GMSCs.
Keywords: Anti-proliferating cell nuclear antigen; Apop-Tag Peroxidase; Guinea pigs; Oral derived stem cells; Xerostomia.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Intraglandular transplantation of bone marrow-derived clonal mesenchymal stem cells for amelioration of post-irradiation salivary gland damage.Oral Oncol. 2013 Feb;49(2):136-43. doi: 10.1016/j.oraloncology.2012.08.010. Epub 2012 Sep 13. Oral Oncol. 2013. PMID: 22981389
-
Adipose-derived mesenchymal stem cells regenerate radioiodine-induced salivary gland damage in a murine model.Sci Rep. 2019 Oct 31;9(1):15752. doi: 10.1038/s41598-019-51775-9. Sci Rep. 2019. PMID: 31673085 Free PMC article.
-
Systemic transplantation of human adipose tissue-derived mesenchymal stem cells for the regeneration of irradiation-induced salivary gland damage.PLoS One. 2013 Aug 9;8(8):e71167. doi: 10.1371/journal.pone.0071167. eCollection 2013. PLoS One. 2013. PMID: 23951100 Free PMC article.
-
Trends in Salivary Gland Tissue Engineering: From Stem Cells to Secretome and Organoid Bioprinting.Tissue Eng Part B Rev. 2021 Apr;27(2):155-165. doi: 10.1089/ten.TEB.2020.0149. Epub 2020 Aug 26. Tissue Eng Part B Rev. 2021. PMID: 32723016 Review.
-
Autologous mesenchymal stem cells offer a new paradigm for salivary gland regeneration.Int J Oral Sci. 2023 May 10;15(1):18. doi: 10.1038/s41368-023-00224-5. Int J Oral Sci. 2023. PMID: 37165024 Free PMC article. Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources

