Distinguishing IDH mutation status in gliomas using FTIR-ATR spectra of peripheral blood plasma indicating clear traces of protein amyloid aggregation
- PMID: 38365669
- PMCID: PMC10870484
- DOI: 10.1186/s12885-024-11970-y
Distinguishing IDH mutation status in gliomas using FTIR-ATR spectra of peripheral blood plasma indicating clear traces of protein amyloid aggregation
Abstract
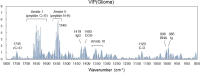
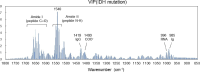
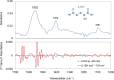
Background: Glioma is a primary brain tumor and the assessment of its molecular profile in a minimally invasive manner is important in determining treatment strategies. Among the molecular abnormalities of gliomas, mutations in the isocitrate dehydrogenase (IDH) gene are strong predictors of treatment sensitivity and prognosis. In this study, we attempted to non-invasively diagnose glioma development and the presence of IDH mutations using multivariate analysis of the plasma mid-infrared absorption spectra for a comprehensive and sensitive view of changes in blood components associated with the disease and genetic mutations. These component changes are discussed in terms of absorption wavenumbers that contribute to differentiation.
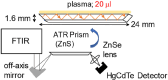
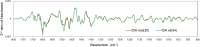
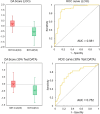
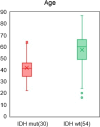
Methods: Plasma samples were collected at our institutes from 84 patients with glioma (13 oligodendrogliomas, 17 IDH-mutant astrocytoma, 7 IDH wild-type diffuse glioma, and 47 glioblastomas) before treatment initiation and 72 healthy participants. FTIR-ATR spectra were obtained for each plasma sample, and PLS discriminant analysis was performed using the absorbance of each wavenumber in the fingerprint region of biomolecules as the explanatory variable. This data was used to distinguish patients with glioma from healthy participants and diagnose the presence of IDH mutations.
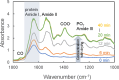
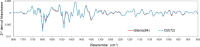
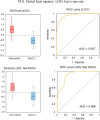
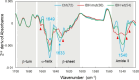
Results: The derived classification algorithm distinguished the patients with glioma from healthy participants with 83% accuracy (area under the curve (AUC) in receiver operating characteristic (ROC) = 0.908) and diagnosed the presence of IDH mutation with 75% accuracy (AUC = 0.752 in ROC) in cross-validation using 30% of the total test data. The characteristic changes in the absorption spectra suggest an increase in the ratio of β-sheet structures in the conformational composition of blood proteins of patients with glioma. Furthermore, these changes were more pronounced in patients with IDH-mutant gliomas.
Conclusions: The plasma infrared absorption spectra could be used to diagnose gliomas and the presence of IDH mutations in gliomas with a high degree of accuracy. The spectral shape of the protein absorption band showed that the ratio of β-sheet structures in blood proteins was significantly higher in patients with glioma than in healthy participants, and protein aggregation was a distinct feature in patients with glioma with IDH mutations.
Keywords: Amyloid; Blood biomarkers; Glioma; IDH mutation; Mid-infrared absorption spectroscopy; Protein aggregation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Noninvasive assessment of isocitrate dehydrogenase mutation status in cerebral gliomas by magnetic resonance spectroscopy in a clinical setting.J Neurosurg. 2018 Feb;128(2):391-398. doi: 10.3171/2016.10.JNS161793. Epub 2017 Mar 3. J Neurosurg. 2018. PMID: 28298040
-
Static 18F-FET PET and DSC-PWI based on hybrid PET/MR for the prediction of gliomas defined by IDH and 1p/19q status.Eur Radiol. 2021 Jun;31(6):4087-4096. doi: 10.1007/s00330-020-07470-9. Epub 2020 Nov 19. Eur Radiol. 2021. PMID: 33211141
-
[Mutation of isocitrate dehydrogenase gene in Chinese patients with glioma].Zhonghua Bing Li Xue Za Zhi. 2013 May;42(5):292-8. doi: 10.3760/cma.j.issn.0529-5807.2013.05.002. Zhonghua Bing Li Xue Za Zhi. 2013. PMID: 24004584 Chinese.
-
Diagnostic accuracy and potential covariates for machine learning to identify IDH mutations in glioma patients: evidence from a meta-analysis.Eur Radiol. 2020 Aug;30(8):4664-4674. doi: 10.1007/s00330-020-06717-9. Epub 2020 Mar 19. Eur Radiol. 2020. PMID: 32193643 Review.
-
Integrated diagnostics of diffuse astrocytic and oligodendroglial tumors.Pathologe. 2019 Jun;40(Suppl 1):9-17. doi: 10.1007/s00292-019-0581-8. Pathologe. 2019. PMID: 31025086 Review. English.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

