This is a preprint.
A programmable arthritis-specific receptor for guided articular cartilage regenerative medicine
- PMID: 38352576
- PMCID: PMC10862827
- DOI: 10.1101/2024.01.31.578281
A programmable arthritis-specific receptor for guided articular cartilage regenerative medicine
Update in
-
A programmable arthritis-specific receptor for guided articular cartilage regenerative medicine.Osteoarthritis Cartilage. 2025 Feb;33(2):231-240. doi: 10.1016/j.joca.2024.12.002. Epub 2024 Dec 18. Osteoarthritis Cartilage. 2025. PMID: 39706287
Abstract
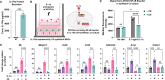
Objective: Investigational cell therapies have been developed as disease-modifying agents for the treatment of osteoarthritis (OA), including those that inducibly respond to inflammatory factors driving OA progression. However, dysregulated inflammatory cascades do not specifically signify the presence of OA. Here, we deploy a synthetic receptor platform that regulates cell behaviors in an arthritis-specific fashion to confine transgene expression to sites characterized by cartilage degeneration.
Methods: An scFv specific for type II collagen (CII) was used to produce a synthetic Notch (synNotch) receptor that enables "CII-synNotch" mesenchymal stromal cells (MSCs) to recognize CII fibers exposed in damaged cartilage. Engineered cell activation by both CII-treated culture surfaces and on primary tissue samples was measured via inducible reporter transgene expression. TGFβ3-expressing cells were assessed for cartilage anabolic gene expression via qRT-PCR. In a co-culture with CII-synNotch MSCs engineered to express IL-1Ra, ATDC5 chondrocytes were stimulated with IL-1α, and inflammatory responses of ATDC5s were profiled via qRT-PCR and an NF-κB reporter assay.
Results: CII-synNotch MSCs are highly responsive to CII, displaying activation ranges over 40-fold in response to physiologic CII inputs. CII-synNotch cells exhibit the capacity to distinguish between healthy and damaged cartilage tissue and constrain transgene expression to regions of exposed CII fibers. Receptor-regulated TGFβ3 expression resulted in upregulation of Acan and Col2a1 in MSCs, and inducible IL-1Ra expression by engineered CII-synNotch MSCs reduced pro-inflammatory gene expression in chondrocytes.
Conclusion: This work demonstrates proof-of-concept that the synNotch platform guides MSCs for spatially regulated, disease-dependent delivery of OA-relevant biologic drugs.
Figures




Similar articles
-
A programmable arthritis-specific receptor for guided articular cartilage regenerative medicine.Osteoarthritis Cartilage. 2025 Feb;33(2):231-240. doi: 10.1016/j.joca.2024.12.002. Epub 2024 Dec 18. Osteoarthritis Cartilage. 2025. PMID: 39706287
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Comparing Two Approaches to Help Patients and Doctors Talk about Chronic Pain Treatments -- Voices in Pain Care [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2022 Apr. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2022 Apr. PMID: 39661719 Free Books & Documents. Review.
-
The effectiveness of abstinence-based and harm reduction-based interventions in reducing problematic substance use in adults who are experiencing homelessness in high income countries: A systematic review and meta-analysis: A systematic review.Campbell Syst Rev. 2024 Apr 21;20(2):e1396. doi: 10.1002/cl2.1396. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38645303 Free PMC article. Review.
References
-
- Woodell-May JE, Sommerfeld SD. Role of Inflammation and the Immune System in the Progression of Osteoarthritis. J Orthop Res. 2020;38(2):253–7. - PubMed
-
- Mehana EE, Khafaga AF, El-Blehi SS. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019;234:116786. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
