Reflections on the ABC model of flower development
- PMID: 38345422
- PMCID: PMC11062442
- DOI: 10.1093/plcell/koae044
Reflections on the ABC model of flower development
Abstract
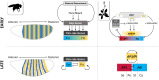
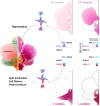
The formulation of the ABC model by a handful of pioneer plant developmental geneticists was a seminal event in the quest to answer a seemingly simple question: how are flowers formed? Fast forward 30 years and this elegant model has generated a vibrant and diverse community, capturing the imagination of developmental and evolutionary biologists, structuralists, biochemists and molecular biologists alike. Together they have managed to solve many floral mysteries, uncovering the regulatory processes that generate the characteristic spatio-temporal expression patterns of floral homeotic genes, elucidating some of the mechanisms allowing ABC genes to specify distinct organ identities, revealing how evolution tinkers with the ABC to generate morphological diversity, and even shining a light on the origins of the floral gene regulatory network itself. Here we retrace the history of the ABC model, from its genesis to its current form, highlighting specific milestones along the way before drawing attention to some of the unsolved riddles still hidden in the floral alphabet.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures




Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article.
-
Australia in 2030: what is our path to health for all?Med J Aust. 2021 May;214 Suppl 8:S5-S40. doi: 10.5694/mja2.51020. Med J Aust. 2021. PMID: 33934362
-
Dynamic Field Theory of Executive Function: Identifying Early Neurocognitive Markers.Monogr Soc Res Child Dev. 2024 Dec;89(3):7-109. doi: 10.1111/mono.12478. Monogr Soc Res Child Dev. 2024. PMID: 39628288 Free PMC article.
-
Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
-
Celebrating the American Society of Plant Biologists centennial anniversary: A compendium of review articles in plant biology.Plant Cell. 2024 May 1;36(5):1183-1185. doi: 10.1093/plcell/koae076. Plant Cell. 2024. PMID: 38466716 Free PMC article. No abstract available.
-
Petal segmentation in CT images based on divide-and-conquer strategy.Front Plant Sci. 2024 Jul 15;15:1389902. doi: 10.3389/fpls.2024.1389902. eCollection 2024. Front Plant Sci. 2024. PMID: 39077510 Free PMC article.
-
The overall regulatory network and contributions of ABC(D)E model genes in yellowhorn flower development.BMC Plant Biol. 2024 Nov 15;24(1):1081. doi: 10.1186/s12870-024-05796-w. BMC Plant Biol. 2024. PMID: 39543490 Free PMC article.
-
'Organ'ising Floral Organ Development.Plants (Basel). 2024 Jun 8;13(12):1595. doi: 10.3390/plants13121595. Plants (Basel). 2024. PMID: 38931027 Free PMC article. Review.
-
Celebrating the American Society of Plant Biologists centennial anniversary: A compendium of review articles in plant biology.Plant Physiol. 2024 Apr 30;195(1):1-3. doi: 10.1093/plphys/kiae141. Plant Physiol. 2024. PMID: 38547371 Free PMC article. No abstract available.
References
-
- Alvarez-Buylla ER, Ambrose BA, Flores-Sandoval E, Vergara-Silva F, Englund M, Garay-Arroyo A, Garcia-Ponce B, de la Torre-Barcena E, Espinosa-Matias S, Martinez E, et al. . B-function expression in the flower center underlies the homeotic phenotype of Lacandonia schismatica (Triuridaceae). Plant Cell. 2010:22(11):3543–3559. 10.1105/tpc.109.069153 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

