Analyzing the Effects of Single Nucleotide Polymorphisms on hnRNPA2/B1 Protein Stability and Function: Insights for Anticancer Therapeutic Design
- PMID: 38343990
- PMCID: PMC10851241
- DOI: 10.1021/acsomega.3c07195
Analyzing the Effects of Single Nucleotide Polymorphisms on hnRNPA2/B1 Protein Stability and Function: Insights for Anticancer Therapeutic Design
Abstract
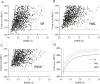
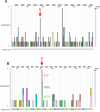
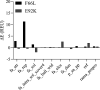
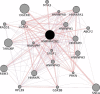
Heterogeneous nuclear ribonucleoprotein A2/B1 (hnRNPA2/B1) is a pivotal player in m6A recognition, RNA metabolism, and antiviral responses. In the context of cancer, overexpression of hnRNPA2/B1, abnormal RNA levels, and m6A depositions are evident. This study focuses on two significant nonsynonymous single nucleotide polymorphisms (nsSNPs) within hnRNPA2/B1, namely, F66L and E92K. Our structural analyses reveal decreased stability in these mutants, with E92K being predicted to undergo destabilizing post-translational methylation. Furthermore, our extensive analysis of 44,239 tumor samples from the COSMIC database uncovers that amino acid position 92 exhibits the second-highest mutation frequency within hnRNPA2/B1, particularly associated with breast and lung cancers. This experimental data aligns with our theoretical studies, highlighting the substantial impact of the nsSNP at position 92 on hnRNPA2/B1's stability and functionality. Given the critical role of pre-mRNA splicing, transcription, and translation regulation in cellular function, it is important to assess the impact of these nsSNPs on the stability and function of the hnRNPA2/B1 protein to design more efficient anticancer therapeutics.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Heterogeneous nuclear ribonucleoprotein A2/B1 is a negative regulator of human breast cancer metastasis by maintaining the balance of multiple genes and pathways.EBioMedicine. 2020 Jan;51:102583. doi: 10.1016/j.ebiom.2019.11.044. Epub 2020 Jan 3. EBioMedicine. 2020. PMID: 31901866 Free PMC article.
-
Cholinergic Regulation of hnRNPA2/B1 Translation by M1 Muscarinic Receptors.J Neurosci. 2016 Jun 8;36(23):6287-96. doi: 10.1523/JNEUROSCI.4614-15.2016. J Neurosci. 2016. PMID: 27277805 Free PMC article.
-
Functional roles of hnRNPA2/B1 regulated by METTL3 in mammalian embryonic development.Sci Rep. 2019 Jun 14;9(1):8640. doi: 10.1038/s41598-019-44714-1. Sci Rep. 2019. PMID: 31201338 Free PMC article.
-
The roles of hnRNP A2/B1 in RNA biology and disease.Wiley Interdiscip Rev RNA. 2021 Mar;12(2):e1612. doi: 10.1002/wrna.1612. Epub 2020 Jun 26. Wiley Interdiscip Rev RNA. 2021. PMID: 32588964 Review.
-
The human mitochondrial transport/carrier protein family. Nonsynonymous single nucleotide polymorphisms (nsSNPs) and mutations that lead to human diseases.Biochim Biophys Acta. 2006 Sep-Oct;1757(9-10):1263-70. doi: 10.1016/j.bbabio.2006.05.024. Epub 2006 May 22. Biochim Biophys Acta. 2006. PMID: 16843431 Review.
Cited by
-
Lipid metabolism disorder in diabetic kidney disease.Front Endocrinol (Lausanne). 2024 Apr 29;15:1336402. doi: 10.3389/fendo.2024.1336402. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38742197 Free PMC article. Review.
References
-
- Wang J.; Sun D.; Wang M.; Cheng A.; Zhu Y.; Mao S.; Ou X.; Zhao X.; Huang J.; Gao Q.; Zhang S.; Yang Q.; Wu Y.; Zhu D.; Jia R.; Chen S.; Liu M. Multiple functions of heterogeneous nuclear ribonucleoproteins in the positive single-stranded RNA virus life cycle. Front. Immunol. 2022, 13, 98929810.3389/fimmu.2022.989298. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
