The Interplay between Heat Shock Proteins and Cancer Pathogenesis: A Novel Strategy for Cancer Therapeutics
- PMID: 38339390
- PMCID: PMC10854888
- DOI: 10.3390/cancers16030638
The Interplay between Heat Shock Proteins and Cancer Pathogenesis: A Novel Strategy for Cancer Therapeutics
Abstract
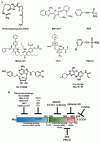
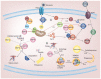
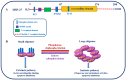
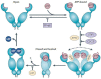
Heat shock proteins (HSPs) are developmentally conserved families of protein found in both prokaryotic and eukaryotic organisms. HSPs are engaged in a diverse range of physiological processes, including molecular chaperone activity to assist the initial protein folding or promote the unfolding and refolding of misfolded intermediates to acquire the normal or native conformation and its translocation and prevent protein aggregation as well as in immunity, apoptosis, and autophagy. These molecular chaperonins are classified into various families according to their molecular size or weight, encompassing small HSPs (e.g., HSP10 and HSP27), HSP40, HSP60, HSP70, HSP90, and the category of large HSPs that include HSP100 and ClpB proteins. The overexpression of HSPs is induced to counteract cell stress at elevated levels in a variety of solid tumors, including anticancer chemotherapy, and is closely related to a worse prognosis and therapeutic resistance to cancer cells. HSPs are also involved in anti-apoptotic properties and are associated with processes of cancer progression and development, such as metastasis, invasion, and cell proliferation. This review outlines the previously mentioned HSPs and their significant involvement in diverse mechanisms of tumor advancement and metastasis, as well as their contribution to identifying potential targets for therapeutic interventions.
Keywords: HSP inhibitors; apoptosis; cancer; chemosensitizing agent; heat shock proteins; molecular chaperones.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities.MedComm (2020). 2022 Aug 2;3(3):e161. doi: 10.1002/mco2.161. eCollection 2022 Sep. MedComm (2020). 2022. PMID: 35928554 Free PMC article. Review.
-
Heat Shock Proteins: Agents of Cancer Development and Therapeutic Targets in Anti-Cancer Therapy.Cells. 2019 Dec 24;9(1):60. doi: 10.3390/cells9010060. Cells. 2019. PMID: 31878360 Free PMC article. Review.
-
Heat Shock Proteins in Cancer Immunotherapy.J Oncol. 2019 Dec 11;2019:3267207. doi: 10.1155/2019/3267207. eCollection 2019. J Oncol. 2019. PMID: 31885572 Free PMC article. Review.
-
The role of heat shock proteins in cancer.Cancer Lett. 2015 May 1;360(2):114-8. doi: 10.1016/j.canlet.2015.02.026. Epub 2015 Feb 23. Cancer Lett. 2015. PMID: 25721081 Review.
-
Heat shock proteins in oncology: diagnostic biomarkers or therapeutic targets?Biochim Biophys Acta. 2011 Dec;1816(2):89-104. doi: 10.1016/j.bbcan.2011.05.001. Epub 2011 May 14. Biochim Biophys Acta. 2011. PMID: 21605630 Review.
Cited by
-
Biogenically synthesized green silver nanoparticles exhibit antimalarial activity.Discov Nano. 2024 Aug 31;19(1):136. doi: 10.1186/s11671-024-04098-2. Discov Nano. 2024. PMID: 39217276 Free PMC article.
-
Impact of Polyethylene Terephthalate Microplastics on Drosophila melanogaster Biological Profiles and Heat Shock Protein Levels.Biology (Basel). 2024 Apr 25;13(5):293. doi: 10.3390/biology13050293. Biology (Basel). 2024. PMID: 38785774 Free PMC article.
-
Mixtures of Three Mortaparibs with Enhanced Anticancer, Anti-Migration, and Antistress Activities: Molecular Characterization in p53-Null Cancer Cells.Cancers (Basel). 2024 Jun 17;16(12):2239. doi: 10.3390/cancers16122239. Cancers (Basel). 2024. PMID: 38927944 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

