Proanthocyanidins-Based Synbiotics as a Novel Strategy for Nonalcoholic Fatty Liver Disease (NAFLD) Risk Reduction
- PMID: 38338453
- PMCID: PMC10856248
- DOI: 10.3390/molecules29030709
Proanthocyanidins-Based Synbiotics as a Novel Strategy for Nonalcoholic Fatty Liver Disease (NAFLD) Risk Reduction
Abstract
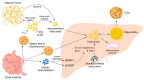
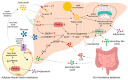
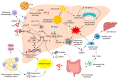
Nonalcoholic fatty liver disease (NAFLD), the most common liver disease worldwide, is a spectrum of liver abnormalities ranging from steatosis to nonalcoholic steatohepatitis (NASH) characterized by excessive lipid accumulation. The prevalence of NAFLD is predicted to increase rapidly, demanding novel approaches to reduce the global NAFLD burden. Flavonoids, the most abundant dietary polyphenols, can reduce the risk of NAFLD. The majority of dietary flavonoids are proanthocyanidins (PACs), which are oligomers and polymers of the flavonoid sub-group flavan-3-ols. The efficacy of PAC in reducing the NAFLD risk can be significantly hindered by low bioavailability. The development of synbiotics by combining PAC with probiotics may increase effectiveness against NAFLD by biotransforming PAC into bioavailable metabolites. PAC and probiotic bacteria are capable of mitigating steatosis primarily through suppressing de novo lipogenesis and promoting fatty acid β-oxidation. PAC and probiotic bacteria can reduce the progression of steatosis to NASH mainly through ameliorating hepatic damage and inflammation induced by hepatic oxidative stress, endoplasmic reticulum stress, and gut microbiota dysbiosis. Synbiotics of PAC are superior in reducing the risk of NAFLD compared to independent administration of PAC and probiotics. The development of PAC-based synbiotics can be a novel strategy to mitigate the increasing incidence of NAFLD.
Keywords: flavonoids; nonalcoholic fatty liver disease; nonalcoholic steatohepatitis; proanthocyanidins; probiotic bacteria; steatosis; synbiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Role of Probiotics in Non-alcoholic Fatty Liver Disease: Does Gut Microbiota Matter?Nutrients. 2019 Nov 19;11(11):2837. doi: 10.3390/nu11112837. Nutrients. 2019. PMID: 31752378 Free PMC article. Review.
-
The Effects of Probiotics, Prebiotics and Synbiotics in Non-Alcoholic Fat Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH): A Systematic Review.Int J Mol Sci. 2022 Aug 8;23(15):8805. doi: 10.3390/ijms23158805. Int J Mol Sci. 2022. PMID: 35955942 Free PMC article. Review.
-
Gut microbiome-targeted therapies in nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression.Am J Clin Nutr. 2019 Jul 1;110(1):139-149. doi: 10.1093/ajcn/nqz042. Am J Clin Nutr. 2019. PMID: 31124558 Free PMC article.
-
Review article: can bugs be drugs? The potential of probiotics and prebiotics as treatment for non-alcoholic fatty liver disease.Aliment Pharmacol Ther. 2019 Sep;50(6):628-639. doi: 10.1111/apt.15416. Epub 2019 Aug 2. Aliment Pharmacol Ther. 2019. PMID: 31373710 Review.
-
Gut microbiota: novel therapeutic target for nonalcoholic fatty liver disease.Expert Rev Gastroenterol Hepatol. 2019 Mar;13(3):193-204. doi: 10.1080/17474124.2019.1569513. Epub 2019 Jan 25. Expert Rev Gastroenterol Hepatol. 2019. PMID: 30791767 Review.
Cited by
-
NAFLD/MASLD and the Gut-Liver Axis: From Pathogenesis to Treatment Options.Metabolites. 2024 Jun 28;14(7):366. doi: 10.3390/metabo14070366. Metabolites. 2024. PMID: 39057689 Free PMC article. Review.
-
Metabolic-Associated Fatty Liver Disease: The Influence of Oxidative Stress, Inflammation, Mitochondrial Dysfunctions, and the Role of Polyphenols.Pharmaceuticals (Basel). 2024 Oct 10;17(10):1354. doi: 10.3390/ph17101354. Pharmaceuticals (Basel). 2024. PMID: 39458995 Free PMC article. Review.
-
Effects of Phenolic Acids Produced from Food-Derived Flavonoids and Amino Acids by the Gut Microbiota on Health and Disease.Molecules. 2024 Oct 29;29(21):5102. doi: 10.3390/molecules29215102. Molecules. 2024. PMID: 39519743 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

