A Randomized Controlled Trial to Evaluate the Impact of a Novel Probiotic and Nutraceutical Supplement on Pruritic Dermatitis and the Gut Microbiota in Privately Owned Dogs
- PMID: 38338095
- PMCID: PMC10854619
- DOI: 10.3390/ani14030453
A Randomized Controlled Trial to Evaluate the Impact of a Novel Probiotic and Nutraceutical Supplement on Pruritic Dermatitis and the Gut Microbiota in Privately Owned Dogs
Abstract
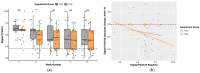
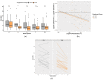
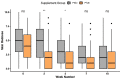
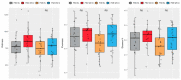
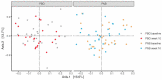
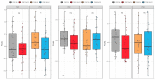
Pruritic dermatitis (PD) is a common presentation of canine allergic skin diseases, with diversity in severity and treatment response due to complex etiopathogenesis. Evidence suggests the gut microbiota (GM) may contribute to the development of canine allergies. A 10-week double-blind randomized controlled trial evaluated a novel probiotic and nutraceutical blend (PNB) on clinical signs of skin allergy, health measures, and the GM of privately owned self-reported pruritic dogs. A total of 105 dogs were enrolled, with 62 included in pruritus and health analysis and 50 in microbiome analysis. The PNB supported greater improvement of owner-assessed clinical signs of PD at week 2 than the placebo (PBO). More dogs that received the PNB shifted to normal pruritus (digital PVAS10-N: <2) by week 4, compared to week 7 for the PBO. While a placebo effect was identified, clinical differences were supported by changes in the GM. The PNB enriched three probiotic bacteria and reduced abundances of species associated with negative effects. The PBO group demonstrated increased abundances of pathogenic species and reduced abundances of several beneficial species. This trial supports the potential of the PNB as a supplemental intervention in the treatment of PD; however, further investigation is warranted, with stricter diagnostic criteria, disease biomarkers and direct veterinary examination.
Keywords: dog; fecal microbiota; pruritic dermatitis; pruritus; skin allergies.
Conflict of interest statement
D.E.T., J.T., R.B.J., and R.W.H. were employees of NomNomNow Inc. at the time the study was completed but are currently employed by Mars Petcare (Waltham Petcare Science Institute). J.S. received compensation from NomNomNow Inc. during data collection and H.M. was contracted as a consultant. A.C., E.K. and S.A.N. are/were employed by Cargill Inc.
Figures

Similar articles
-
Randomized, double-blinded, placebo-controlled pilot study on the effects of topical blackcurrant emulsion enriched in essential fatty acids, ceramides and 18-beta glycyrrhetinic acid on clinical signs and skin barrier function in dogs with atopic dermatitis.Vet Dermatol. 2017 Dec;28(6):577-e140. doi: 10.1111/vde.12467. Epub 2017 Jul 23. Vet Dermatol. 2017. PMID: 28736984 Clinical Trial.
-
Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis.Vet Dermatol. 2013 Oct;24(5):479-e114. doi: 10.1111/vde.12047. Epub 2013 Jul 5. Vet Dermatol. 2013. PMID: 23829933 Free PMC article. Clinical Trial.
-
Clinical efficacy of low-level laser therapy on localized canine atopic dermatitis severity score and localized pruritic visual analog score in pedal pruritus due to canine atopic dermatitis.Vet Dermatol. 2014 Oct;25(5):464-e74. doi: 10.1111/vde.12144. Epub 2014 Jun 9. Vet Dermatol. 2014. PMID: 24909192
-
A blinded, randomized, placebo-controlled, dose determination trial of lokivetmab (ZTS-00103289), a caninized, anti-canine IL-31 monoclonal antibody in client owned dogs with atopic dermatitis.Vet Dermatol. 2016 Dec;27(6):478-e129. doi: 10.1111/vde.12376. Epub 2016 Sep 19. Vet Dermatol. 2016. PMID: 27647569 Clinical Trial.
-
Canine dermatitis on contacting grass leaf: A case series.Vet Dermatol. 2023 Apr;34(2):115-124. doi: 10.1111/vde.13143. Epub 2023 Jan 12. Vet Dermatol. 2023. PMID: 36635786 Review.
References
-
- Nagle T.M., Torres S.M., Horne K.L., Grover R., Stevens M.T. A Randomized, Double-Blind, Placebo-Controlled Trial to Investigate the Efficacy and Safety of a Chinese Herbal Product (P07P) for the Treatment of Canine Atopic Dermatitis. Vet. Dermatol. 2001;12:265–274. doi: 10.1046/j.0959-4493.2001.00267.x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

