The Opioid Receptor Influences Circadian Rhythms in Human Keratinocytes through the β-Arrestin Pathway
- PMID: 38334624
- PMCID: PMC10854934
- DOI: 10.3390/cells13030232
The Opioid Receptor Influences Circadian Rhythms in Human Keratinocytes through the β-Arrestin Pathway
Abstract
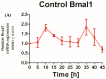
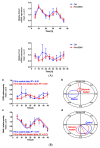
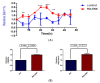
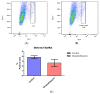
The recent emphasis on circadian rhythmicity in critical skin cell functions related to homeostasis, regeneration and aging has shed light on the importance of the PER2 circadian clock gene as a vital antitumor gene. Furthermore, delta-opioid receptors (DOPrs) have been identified as playing a crucial role in skin differentiation, proliferation and migration, which are not only essential for wound healing but also contribute to cancer development. In this study, we propose a significant association between cutaneous opioid receptor (OPr) activity and circadian rhythmicity. To investigate this link, we conducted a 48 h circadian rhythm experiment, during which RNA samples were collected every 5 h. We discovered that the activation of DOPr by its endogenous agonist Met-Enkephalin in N/TERT-1 keratinocytes, synchronized by dexamethasone, resulted in a statistically significant 5.6 h delay in the expression of the core clock gene PER2. Confocal microscopy further confirmed the simultaneous nuclear localization of the DOPr-β-arrestin-1 complex. Additionally, DOPr activation not only enhanced but also induced a phase shift in the rhythmic binding of β-arrestin-1 to the PER2 promoter. Furthermore, we observed that β-arrestin-1 regulates the transcription of its target genes, including PER2, by facilitating histone-4 acetylation. Through the ChIP assay, we determined that Met-Enkephalin enhances β-arrestin-1 binding to acetylated H4 in the PER2 promoter. In summary, our findings suggest that DOPr activation leads to a phase shift in PER2 expression via β-arrestin-1-facilitated chromatin remodeling. Consequently, these results indicate that DOPr, much like its role in wound healing, may also play a part in cancer development by influencing PER2.
Keywords: beta-arrestin; circadian rhythm; keratinocyte; opioid receptor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Activation of the δ-opioid receptor promotes cutaneous wound healing by affecting keratinocyte intercellular adhesion and migration.Br J Pharmacol. 2015 Jan;172(2):501-14. doi: 10.1111/bph.12687. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24628261 Free PMC article.
-
Transcription Factor EGR1 Regulates the Expression of the Clock Gene PER2 under IL-4 Stimulation in Human Keratinocytes.J Invest Dermatol. 2022 Oct;142(10):2677-2686.e9. doi: 10.1016/j.jid.2022.03.021. Epub 2022 Apr 7. J Invest Dermatol. 2022. PMID: 35398375
-
Phosphorylation of the delta-opioid receptor regulates its beta-arrestins selectivity and subsequent receptor internalization and adenylyl cyclase desensitization.J Biol Chem. 2007 Aug 3;282(31):22315-23. doi: 10.1074/jbc.M611258200. Epub 2007 Jun 12. J Biol Chem. 2007. PMID: 17565992
-
Alterations of circadian clockworks during differentiation and apoptosis of rat ovarian cells.Chronobiol Int. 2011 Jul;28(6):477-87. doi: 10.3109/07420528.2011.589933. Chronobiol Int. 2011. PMID: 21797776
-
Induction of delta-opioid receptor function in the midbrain after chronic morphine treatment.J Neurosci. 2005 Mar 23;25(12):3192-8. doi: 10.1523/JNEUROSCI.4585-04.2005. J Neurosci. 2005. PMID: 15788776 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

