Insights into innate immune cell evasion by Chlamydia trachomatis
- PMID: 38333214
- PMCID: PMC10850350
- DOI: 10.3389/fimmu.2024.1289644
Insights into innate immune cell evasion by Chlamydia trachomatis
Abstract
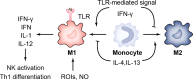
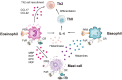
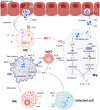
Chlamydia trachomatis, is a kind of obligate intracellular pathogen. The removal of C. trachomatis relies primarily on specific cellular immunity. It is currently considered that CD4+ Th1 cytokine responses are the major protective immunity against C. trachomatis infection and reinfection rather than CD8+ T cells. The non-specific immunity (innate immunity) also plays an important role in the infection process. To survive inside the cells, the first process that C. trachomatis faces is the innate immune response. As the "sentry" of the body, mast cells attempt to engulf and remove C. trachomatis. Dendritic cells present antigen of C. trachomatis to the "commanders" (T cells) through MHC-I and MHC-II. IFN-γ produced by activated T cells and natural killer cells (NK) further activates macrophages. They form the body's "combat troops" and produce immunity against C. trachomatis in the tissues and blood. In addition, the role of eosinophils, basophils, innate lymphoid cells (ILCs), natural killer T (NKT) cells, γδT cells and B-1 cells should not be underestimated in the infection of C. trachomatis. The protective role of innate immunity is insufficient, and sexually transmitted diseases (STDs) caused by C. trachomatis infections tend to be insidious and recalcitrant. As a consequence, C. trachomatis has developed a unique evasion mechanism that triggers inflammatory immunopathology and acts as a bridge to protective to pathological adaptive immunity. This review focuses on the recent advances in how C. trachomatis evades various innate immune cells, which contributes to vaccine development and our understanding of the pathophysiologic consequences of C. trachomatis infection.
Keywords: Chlamydia trachomatis; immune evasion; innate immune cells; innate immunity; survival and growth.
Copyright © 2024 Wang, Wu, Fang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The Predominant CD4+ Th1 Cytokine Elicited to Chlamydia trachomatis Infection in Women Is Tumor Necrosis Factor Alpha and Not Interferon Gamma.Clin Vaccine Immunol. 2017 Apr 5;24(4):e00010-17. doi: 10.1128/CVI.00010-17. Print 2017 Apr. Clin Vaccine Immunol. 2017. PMID: 28100498 Free PMC article.
-
Innate Lymphoid Cells Are Required for Endometrial Resistance to Chlamydia trachomatis Infection.Infect Immun. 2020 Jun 22;88(7):e00152-20. doi: 10.1128/IAI.00152-20. Print 2020 Jun 22. Infect Immun. 2020. PMID: 32341118 Free PMC article.
-
T cell responses to Chlamydia.Pathog Dis. 2021 Mar 31;79(4):ftab014. doi: 10.1093/femspd/ftab014. Pathog Dis. 2021. PMID: 33693620 Free PMC article. Review.
-
Humoral and cellular immunity in secondary infection due to murine Chlamydia trachomatis.Infect Immun. 1997 Jul;65(7):2876-82. doi: 10.1128/iai.65.7.2876-2882.1997. Infect Immun. 1997. PMID: 9199462 Free PMC article.
-
Immunity, immunopathology, and human vaccine development against sexually transmitted Chlamydia trachomatis.Hum Vaccin Immunother. 2014;10(9):2664-73. doi: 10.4161/hv.29683. Hum Vaccin Immunother. 2014. PMID: 25483666 Free PMC article. Review.
Cited by
-
Immunity to Sexually Transmitted Bacterial Infections of the Female Genital Tract: Toward Effective Vaccines.Vaccines (Basel). 2024 Aug 1;12(8):863. doi: 10.3390/vaccines12080863. Vaccines (Basel). 2024. PMID: 39203989 Free PMC article. Review.
-
Research progress on V delta 1+ T cells and their effect on pathogen infection.PeerJ. 2024 Oct 30;12:e18313. doi: 10.7717/peerj.18313. eCollection 2024. PeerJ. 2024. PMID: 39494290 Free PMC article. Review.
-
Editorial: Innate immunity against intracellular bacteria: mechanisms and strategies.Front Immunol. 2024 Mar 20;15:1396114. doi: 10.3389/fimmu.2024.1396114. eCollection 2024. Front Immunol. 2024. PMID: 38571948 Free PMC article. No abstract available.
-
Comprehensive single-cell and bulk transcriptomic analyses to develop an NK cell-derived gene signature for prognostic assessment and precision medicine in breast cancer.Front Immunol. 2024 Oct 23;15:1460607. doi: 10.3389/fimmu.2024.1460607. eCollection 2024. Front Immunol. 2024. PMID: 39507529 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

